At presentation, the patient had already undergone an initial serologic evaluation for several forms of infectious retinitis by an outside ophthalmologist. HIV testing was negative, QuantiFERON-TB Gold was negative for tuberculosis and RPR/FTA-ABS tests for syphilis were nonreactive. Serum IgG testing returned positive for HSV-1, CMV and VZV, but IgM testing was negative for all three. Both IgG and IgM testing were negative for HSV-2. Complete blood count, basic metabolic panel and liver function tests were unremarkable.
Fundus photographs and OCT of the macula OU were taken in the office, and can be seen in Figures
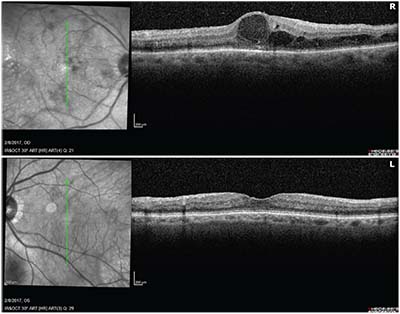 |
| Figure 2. OCT macula of the right and left eyes demonstrating macular thickening and edema on the right. |
The patient returned two weeks later with slight subjective improvement in his vision and fewer floaters. Visual acuity had improved to 20/50 in the right eye. The anterior chamber reaction was improved. Repeat dilated fundus examination revealed less vitreous haze and stability in the area of retinitis OD (See Figure 3). PCR from the anterior chamber sample returned positive for CMV (377,000 copies/ml) and negative for HSV-1 and -2, VZV and Toxoplasma gondii.
Discussion
Diabetes mellitus leads to a state of relative immune compromise through complex mechanisms, including dysfunction of host immune cells and increased virulence of pathogenic organisms in a high-glucose environment.1-3 Although cytomegalovirus (CMV) retinitis is classically associated with acquired immune deficiency syndrome (AIDS) with CD4 counts below 50 cells/ul,4 it has been reported in a variety of other conditions of immune dysfunction. A recent review identified 208 HIV-negative patients who developed CMV retinitis in one or both eyes. Nearly 80 percent of the patients included in the review had a clear cause for immune dysfunction such as malignancy, severe autoimmune condition requiring immunosuppression or a history of bone marrow or solid organ transplantation requiring long-term immunosuppressive therapy.5 However, in 6.1 percent of patients, the only identified cause of immune dysfunction was diabetes. A total of 10.1 percent of patients developed CMV retinitis after injection of intraocular or periocular corticosteroids. Intraocular corticosteroids are a therapeutic option for diabetic macular edema, but they also lead to local immunosuppression within the eye. The patient presented here received multiple intraocular injections of corticosteroids that may have contributed to a degree of local immunosuppression sufficient for the development of CMV retinitis.
Cystoid macular edema is a frequent cause of decreased vision among patients with diabetes, but it is rarely encountered in patients with AIDS-related CMV retinitis.6-9 Diabetes likely contributed to the macular edema seen in this case, but it may not have been the only causative factor. In contrast to AIDS-related CMV retinitis, HIV-negative patients with CMV retinitis may develop CME. HIV-negative patients with CMV retinitis develop more pronounced intraocular inflammation in response to the infection, and it is theorized that this inflammation may lead to the development of macular edema.9 With treatment of the CMV retinitis, the CME seen in this case improved, though it did not completely resolve.
While medications active against vascular endothelial growth factor (VEGF) have become the first-line intravitreal pharmacologic agent in the treatment of diabetic macular edema based on the results of several large trials demonstrating their efficacy,10-12 as previously
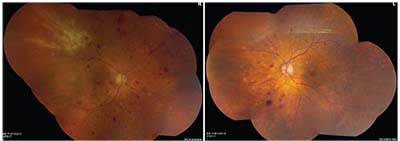 |
| Figure 3. Repeat montage fundus photographs of both eyes showing less vitreous haze and stability of the retinitis OD after two weeks of oral valganciclovir therapy for CMV retinitis. |
In the patient presented, relative systemic immune compromise from diabetes as well as local immune suppression from intravitreal corticosteroid injections likely contributed to the development of CMV retinitis. CMV retinitis should be kept in the differential diagnosis when the typical funduscopic findings of the infection are seen, even among patients without HIV who are traditionally described as immunocompetent. The risk of intraocular infection should be considered in patients receiving repeated intravitreal corticosteroid injections. REVIEW
1. Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol 1999;26:256–65.
2. Muller LM, Gorter KJ, Hak E, Goudzwaard WL, Schellevis FG, Hoepelman AI, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis 2005;41:281–8.
3. Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: Pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev 2007;23:3–13.
4. Cunningham ET. Cytomegalovirus: Ophthalmic perspectives on a pervasive pathogen. Expert Rev Ophthalmol 2011;6:489-491.
5. Downes KM, Tarasewicz D, Weisberg LJ, Cunningham ET. Good syndrome and other causes of cytomegalovirus retinitis in HIV-negative patients—case report and comprehensive review of the literature. Journal of Ophthalmic Inflammation and Infection 2016;6:3.
6. Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy IV: Diabetic macular edema. Ophthalmology 1984;91:1464–74.
7. Diabetes Control and Complication Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86.
8. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–53.
9. Maguire AM, Nichols CW, Crooks GW. Visual loss in cytomegalovirus retinitis caused by cystoid macular edema in patients without the acquired immune deficiency syndrome. Ophthalmology 1996;103:601-605.
10. The Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117:1064-1077.
11. Elman MJ, Ayala A, Bressler NM, et al. Intravitreal ranibizumab for diabetic macular edema with prompt vs. deferred laser treatment: 5-year randomized trial results. Ophthalmology 2015;122:375-381.
12. The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Eng J Med 2015;372:1193-1203.
13. VanderBeek BL, Bonaffini SG, Ma L. The association between intravitreal steroids and post-injection endophthalmitis rates. Ophthalmology 2015;122:11:2311-2315.e2311.



