The State of the Art
Optical coherence tomography is based on the principle of low-coherence interferometry. Light is backscattered from the ocular tissue and compared to that of a reference beam.
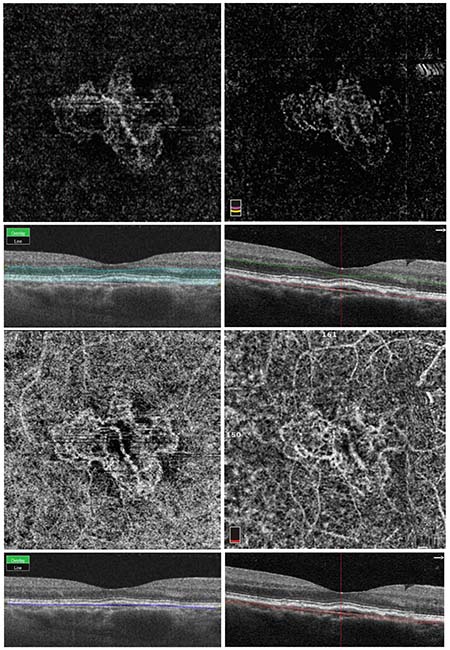 |
| Figure 1. Same-day optical coherence tomography angiography imaging of choroidal neovascularization using swept-source and spectral-domain devices. The top two images are automated segmentation slabs of the outer retina with the corresponding structural cross-sectional OCT-B scan below each image. The bottom two images are automated segmentation slabs at the level of the choriocapillaris. These choriocapillaris images show significant projection artifact of the superficial and deep capillary plexuses due to inclusion of the retinal pigment epithelium in the segmentation slab, making it difficult to visualize the full extent of the neovascular membrane. The images on the left were imaged on the Topcon DRI Triton device, which is a 1050-nm swept-source device operating at a rate of 100,000 A-scans/second. The images on the right were imaged on the OptoVue RTVue XR Avanti, an 840 nm spectral-domain device operating at a rate of 70,000 A-scans/second. |
Spectral-domain and the newer technology known as swept-source OCT are variations of Fourier-domain OCT, in which the interference patterns undergo a process known as Fourier transformation, which allows simultaneous measurement of all light echoes. Spectral-domain devices detect light echoes in the Fourier domain and measure the interference spectrum with a spectrometer and high-speed line scan camera. These systems are able to operate at increased speeds with enhanced sensitivity and signal-to-noise ratios. The sensitivity is enhanced by the ratio of axial resolution to imaging depth. SD-OCT devices are the current standard for ophthalmic instruments, with imaging speeds ranging from 25,000 to 85,000 A-scans per second.
SS-OCT is a variation of Fourier-domain OCT previously known as optical frequency domain reflectometry, which has recently been employed as tomography, and even more recently applied to OCT angiography. The hardware of SS-OCT differs from SD-OCT in several ways, including the light source, bulk optics components and photodetection device. The swept-source light source has a wavelength centered at ~1 µm that sweeps across a narrow band of wavelengths, while spectral-domain devices utilize a broadband light source. The laser frequency sweep labels different time delays, which are then detected by interference. To detect the light waves returning to the device, SS-OCT utilizes a point photodetector, while SD-OCT uses a spectrometer consisting of a diffraction grating, Fourier transform lens, and a detector array or a linescan camera. Although the light source of the SS system is more complex, the photodetector device is simpler in design and results in increased scanning speeds.
The concept of SS-OCT was described in the mid-1990s, but development was limited by the performance of available laser technology, which has since improved with the release of commercially available short-cavity swept lasers with increased scanning speeds. Scanning speeds of up to a million A-scans per second have been achieved with swept-source systems. Increased scanning speeds yield a high-density scan with high resolution en face OCT images, but at the expense of worse signal-to-noise ratio. (There are currently commercially available SS-OCT devices operating at a speed of 100,000 A-scans/second which will be described later in this article.) SS-OCT devices are more prevalent in clinical applications of OCT beyond ophthalmology, including cardiology, dermatology and gastroenterology.
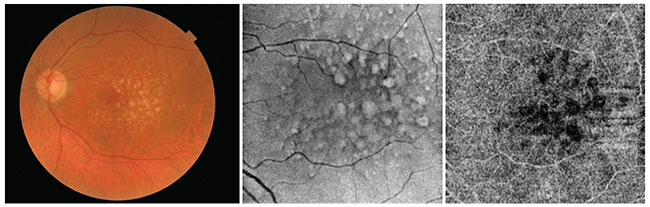 |
| Figure 2. Multimodal imaging of drusen in a patient with non-exudative macular degeneration. The patient was imaged on the swept-source Topcon DRI Triton device. The first image is a color fundus photo taken by the Triton. The second image is the structural en face OCT-B scan, and the third image is an OCTA slab automatically segmented at the level of the choriocapillaris. The dark area underlying the drusen could be an area of low OCT signal secondary to attenuation due to overlying drusen, or areas of choriocapillaris flow impairment, but which one it is can’t be determined based on this image alone. |
In both spectral-domain and swept-source systems, the wavelength of the light source plays an important role in the visualization of retinal and choroidal structures, particularly at deeper locations beneath the RPE. Longer wavelengths are capable of improved visualization of the choriocapillaris and choroid, have improved immunity to ocular opacity, and may be useful for improved visualization of choroidal neovascularization, especially the sub-RPE components of the membrane. However, longer wavelengths have lower resolution compared to shorter ones. Therefore, there’s a trade-off in resolution with increasing bandwidth. In shorter-wavelength systems, attenuation can become more severe in the presence of RPE clumping and drusen or thickened choroid, as in central serous chorioretinopathy.
Advantages and Disadvantages
SD-OCT devices are widely used to evaluate vitreous, retinal and choroidal pathology. Compared to spectral-domain OCT, however, SS devices improve the visualization of structures beneath the RPE due to decreased sensitivity roll-off and attenuation of the OCT signal in deeper structures, particularly the choroid. To overcome this limitation in SD devices, techniques such as enhanced depth imaging are used to better visualize the choroid and structures below the RPE in cross-sectional SD-OCT images. EDI involves image averaging in conjunction with setting the choroid adjacent to the zero-delay line. The zero delay is the axial range position of maximal sensitivity for signal detection.
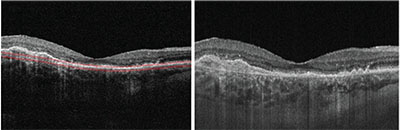 |
| Figure 3. Cross-sectional OCT B-scans from a patient with neovascular age-related macular degeneration. The image on the left is from an 840-nm spectral-domain system operating at a rate of 68,000 A-scans/second, and the image on the right is from a prototype 1050-nm swept-source system at the Massachusetts Institute of Technology operating at a rate of 400,000 A-scans/second. The image from the prototype swept-source device demonstrates reduced signal attenuation and less sensitivity roll-off than that of the spectral domain system. |
Comparisons between swept-source and spectral-domain devices suggest that swept-source devices allow for improved visualization of the choroidal-scleral interface. However, with regards to choroidal thickness
measurements, there are conflicting results in published comparisons, possibly due to the different devices being compared. Though SS-OCT devices tend to have worse axial resolution than SD-OCT, image quality can be improved by software enhancements.
The overall simpler design of SS-OCT devices should enable them to be produced in a more compact form and at lower cost in the future, making them a viable option for further commercial development. Advances in laser technology have enabled the development of SS-OCT technology in recent years, but the current market acceptance of SS-OCT devices is limited by high costs, limited availability and a lack of normative data. Also, the clinical advantages of SS-OCT versus SD aren’t clear; though SS offers improved visualization of the choroid and structures below the RPE, the clinical significance of this remains to be investigated.
Current SS-OCT Devices
• Topcon DRI OCT Triton. Currently, the Topcon Deep Range Imaging OCT Triton is commercially available in Europe and Asia but is only available for research purposes in the United States. It’s an SS device that uses a 1050-nm wavelength light source, has a scanning rate of 100,000 A-scans per second and has an axial resolution of 8 μm. The Triton uses the OCT Angiography Ratio Analysis as its software-based angiography method, which utilizes amplitude information and keeps the full spectrum intact so that the axial resolution is preserved. The device has both conventional OCT, en face OCT and OCTA capabilities. The invisible wavelength of the light source allows patients to fixate on the target during scanning, which reduces involuntary eye movements. The eye-tracking capabilities also reduce motion artifact in these scans.
Combination scan protocols can be used with the Triton, which enable the simultaneous acquisition of a three-dimensional wide-field 12 x 9-mm image, thickness map and cross-sectional OCT-B. Precise localization of cross-sectional OCT-B scans can be obtained using fundus-guided acquisition, which allows the operator to manually select the scan area on the fundus image. The DRI OCT Triton is also unique in its multimodal imaging capabilities: It’s able to acquire color fundus photos, fundus autofluorescence, red-free images and fluorescein angiography. Same-day FA and OCTA scans can be obtained on this device, making it ideal for comparing the two methods for visualizing retinal and choroidal vasculature in vivo. Finally, improved vitreous visualization is possible with features known as Enhanced Vitreous Visualization and Dynamic Focus, which are designed to ensure uniform image quality with uniform focus across the imaging range.
• Zeiss Plex Elite 9000. This was unveiled in May 2016 and is an SS-OCT device introduced for clinical
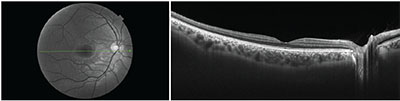 |
| Figure 4. Cross-sectional OCT B-scan from the right eye of a 28-year-old healthy subject. The patient was imaged on the swept-source Topcon DRI Triton device. The image on the left is the red-free fundus image, with the green line corresponding to the cross-sectional OCT-B scan. |
The Plex Elite 9000 makes use of a swept-source, tunable laser centered at 1060 nm and operating at a scan speed of 100,000 A-scans per second with an axial resolution of 6.3 μm. The major difference between the Cirrus HD-OCT system and the Plex Elite SS-OCT system is the use of a tunable laser-based swept source with a Class 1 laser light system and an updated interferometer. The software-based angiography method used in this device is the OMAGc method of processing angiographic data, which uses both phase and amplitude information. It has conventional cross-sectional OCT, en face OCT and OCTA capabilities.
This device also features mechanical eye-tracking capabilities and a 56-degree field of view. This device uses wider OCTA scan protocols than the currently available 3 x 3-mm and 6 x 6-mm scan patterns, and now includes 9 x 9-mm and 12 x 12-mm OCTA protocols. At first glance, the two commercially available swept-source devices appear similar, though differences in image processing software and acquisition scan protocols may lead to differences in the appearance of same-day images taken on the two devices.
• Research prototype devices. Prototype SS-OCT devices have been employed in the research setting for many years. These prototypes are typically tailored for the purposes of investigating a specific research question, and include such technology as anterior segment imaging, wide-field OCTA imaging or ultra high-speed SS-OCT imaging. Images from these prototypes can vary widely and typically require custom image processing software. Therefore, the only definitive similarities between the multiple prototypes that have been referenced in the literature are basic hardware components and the type of light source used.
Future of SS-OCT
OCTA is a relatively new imaging technique utilizing existing OCT technology to noninvasively visualize the retinal and choroidal microvasculature. In OCTA, multiple sequential OCT-B scans are acquired in rapid succession. These sequentially acquired OCT-B scans are compared; the decorrelation signal used to generate the image of the vasculature corresponds to regions
 |
Though currently available OCTA devices use SD-OCT systems, prototype swept-source devices have been used in the research setting to visualize changes in the choriocapillaris in diabetes and age-related macular degeneration. Both spectral-domain and swept-source devices have been used to qualitatively and quantitatively describe the microvascular morphology of choroidal neovascularization.
In the United States, swept-source devices will soon become commercially available, expanding the number of choices available to ophthalmologists when considering OCT systems. Swept-source OCT devices are able to operate at higher scanning speeds than spectral-domain systems and, though their clinical superiority is still unclear, remain promising prospects for future imaging development.
Ms. Cole is a fourth-year medical student at Tufts University School of Medicine and was the 2015-2016 OCT Research Fellow at the New England Eye Center. She can be reached at emilycole10@gmail.com. Dr. Duker is a professor and chairman of ophthalmology at the Tufts Medical Center and Tufts University School of Medicine in Boston. His email is jduker@tuftsmedicalcenter.org
Dr. Duker is a consultant for and receives research support from Carl Zeiss Meditec, OptoVue and Topcon Medical Systems Inc. Ms. Cole has no financial disclosures.
Selected References
1. Fujimoto J, Swanson E. The development, commercialization, and impact of optical coherence tomography. Invest Ophthalmol Vis Sci 2016;57:OCT1-OCT13.
2. Lane M, Moult EM, Novais EA, et al. Visualizing the choriocapillaris under drusen: Comparing 1050-nm swept-source versus 840-nm spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci 2016;57:OCT585-590.
3. Chinn SR, Swanson EA, Fujimoto JG. OCT using a frequency-tunable optical source. Opt Lett 1997;22:340-342.
4. Yasuno Y, Hong Y, Makita S, et al. In vivo high-contrast imaging of deep posterior eye by 1-micron SS-OCT and scattering OCTA. Opt Express 2007;15:6121-6139.
5. Yasuno Y, Madjarova VD, Makita S, et al. Three-dimensional and high-speed swept-source optical coherence tomography for in vivo investigation of human anterior eye segments. Opt Express 2005;13:10652-10664.
6. Choma M, Sarunic M, Yang C, Izatt J. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt Express 2003;11:2183-2189.
7. Ferrara D, Waheed NK, Duker JS. Investigating the choriocapillaris and choroidal vasculature with new OCT technologies. Progress in retinal and eye research 2016;52:130.
8. Saito M, Iida T, Nagayama D. Cross-sectional and en face optical coherence tomographic features of polypoidal choroidal vasculopathy. Retina 2008;28:459-464.
9. Ueno C, Gomi F, Sawa M, Nishida K. Correlation of indocyanine green angiography and optical coherence tomography findings after intravitreal ranibizumab for polypoidal choroidal vasculopathy. Retina 2012;32:2006-2013.
10. Adhi M, Liu JJ, Qavi AH, et al. Choroidal analysis in healthy eyes using swept-source OCT compared to spectral domain OCT. Am J Ophthalmol 2014;157:1272-1281 e1271.
11. Copete S, Flores-Moreno I, Montero JA, Duker JS, Ruiz-Moreno JM. Direct comparison of spectral-domain and swept-source OCT in the measurement of choroidal thickness in normal eyes. Br J Ophthalmol 2014;98:334-338.
12. Matsuo Y, Sakamoto T, Yamashita T, et al. Comparisons of choroidal thickness of normal eyes obtained by two different spectral-domain OCT instruments and one swept-source OCT instrument. Invest Ophthalmol Vis Sci 2013;54:7630-7636.
13. Tan CSH, Ngo WK, Cheong KX. Comparison of choroidal thicknesses using swept source and spectral domain OCT in diseased and normal eyes. Br J Ophthalmol 2015;99:3:354-8.
14. Tatham AJ. New swept-source OCT for glaucoma: Improvements and advantages. Rev of Ophthalmol, Mar 2014.
15. Choi W, Moult EM, Waheed NK, et al. Ultrahigh-speed, swept-source OCTA in nonexudative age-related macular degeneration with geographic atrophy. Ophthalmology 2015;122:2532-2544.



