As myopia reaches epidemic proportions around the globe, clinicians just received a new weapon to aid them in the fight against it: CooperVision received U.S. Food and Drug Administration approval for the MiSight (omafilcon A) daily wear single use soft contact lens, designed for the correction of myopia and for slowing the progression of myopia.
 |
MiSight is indicated for use in children with healthy eyes, who at the initiation of treatment are 8 to 12 years old and have a spherical equivalent refraction between -0.75 and -4 D with no more than 0.75 D of astigmatism.
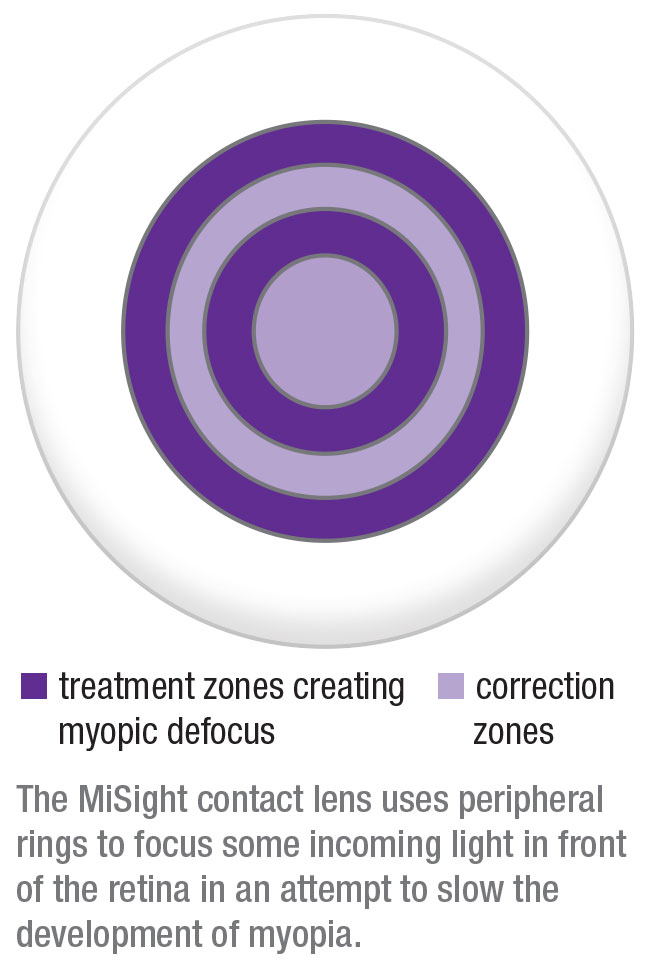
According to an FDA description of the device, when it’s placed on the eye, one part of the MiSight contact lens corrects the refractive error to improve distance vision in nearsighted eyes, while concentric peripheral rings in the lens focus part of the light in front of the retina. This latter effect is believed to reduce the stimulus causing the progression of myopia.
The approval of MiSight was based on data obtained from a prospective clinical trial at four clinical sites and “real-world evidence.” The trial showed that over a three-year period, the progression in myopia of children wearing MiSight lenses was less than those wearing conventional soft contact lenses. In addition, subjects who used MiSight had less change in the axial length of the eyeball at each annual checkup. Over the course of the trial, there were no serious ocular adverse events in either arm of the study, and the rate of corneal ulcers was “comparable” to that in adults who wear daily contact lenses. The company says the lens will be available in spring of 2020, and, as part of the approval of MiSight, it will conduct a postmarket study of the contact lenses to further evaluate the safety and effectiveness of the product. For information, visit https://coopervision.com/contact-lenses/misight-1-day.
Optos Unveils Silverstone
If you’ve been looking for an ultra-widefield optical coherence tomographer that uses swept-source technology, Optos has a new device for you. The company recently launched Silverstone, an imaging system combining ultra-widefield retinal imaging with integrated, image-guided, swept-source OCT.
Optos says that Silverstone produces a 200-degree, single-capture Optomap image with guided OCT, enabling OCT imaging anywhere across the retina, from posterior pole to the far periphery. It has features such as a 3-in-1 Color Depth Imaging, which provides clinical data from the retinal surface through the choroid, Optos says. For more information, visit https://www.optos.com/en/products/silverstone.
Mini but Mighty
If your AMD patients have trouble swallowing normal-sized gels or tablets, Bausch + Lomb may have a solution: PreserVision AREDS 2 Formula mini-gels, which will replace the previously offered soft gels. The company says that the vitamins contain the same National Eye Institute-recommended formula for moderate to advanced age-related macular degeneration.
For information on the new gels, visit https://www.bausch.com/our-products/eye-vitamins/age-related-eye-vitamins. REVIEW



