 |
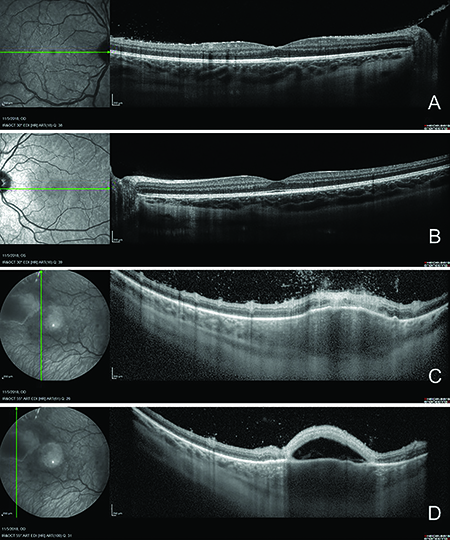
| Figure 2. OCT of the right eye revealed (A) disruption of the inner and outer retina with subtle cystic changes and mild vitritis in the temporal margin. The left macula (B) was normal. OCT through the inflammatory mass in the right eye (C,D) revealed overlying vitritis, retinitis, choroiditis and subretinal fluid with debris. |
Workup, Diagnosis and Treatment
Imaging of the affected eye with macular OCT (Fig. 2A) revealed disruption of the inner and outer retina with subtle cystic changes and mild vitritis in the temporal macula. OCT through the inflammatory mass in the right eye revealed overlying vitritis, retinitis, choroiditis and subretinal fluid with debris (Figure 2C, D). B-scan ultrasonography (Figure 3) revealed an echodense elevated mass measuring 4.26 mm in diameter by 0.91 mm in thickness. The left macula was normal.
Based on the appearance of the retinal lesion, the patient was diagnosed with Toxoplasma chorioretinitis; the patient and his parents denied any risk factors for exposure including contact with cats or raw meat/unclean foods. He was treated empirically with Bactrim while awaiting serologic testing results. Unfortunately, the first collected sample was inadequate and a second sample had to be drawn after he had already undergone two weeks of treatment. Titers for Toxoplasma IgG were positive at 164 IU/mL, but the IgM was negative. Although it wasn’t possible to confirm active Toxoplasma infection with this particular test, the patient was treated empirically with Bactrim, prednisolone acetate 1% and dorzolamide 2%-timolol 0.5% for intraocular pressure control during the early inflammatory phase. He improved over the course of six weeks.
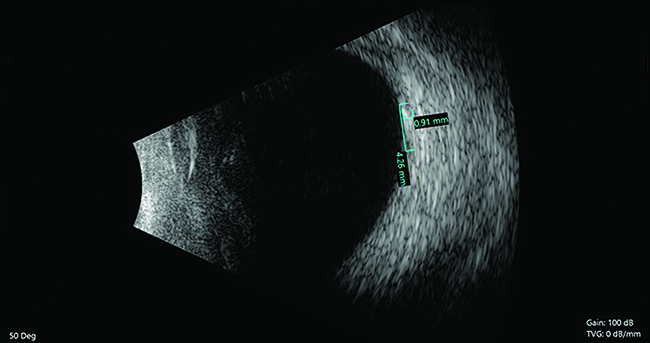 |
| Figure 3. B-scan ultrasonography revealed an echodense elevated mass measuring 4.26 mm in diameter and 0.91 mm thick. |
Discussion
The differential diagnosis in this young patient with acute onset, unilateral decreased vision and photophobia in the setting of ocular hypertension and an inflammatory retinal lesion included infectious, inflammatory and neoplastic etiologies. Infectious causes included protozoan (e.g., toxoplasmosis), bacterial (e.g., Bartonella, tuberculosis, syphilis, Lyme disease), viral (e.g., mumps, herpes simplex), fungal (e.g., histoplasmosis) and nematodal (e.g., Toxocara). Inflammatory etiologies included juvenile idiopathic arthritis, sarcoidosis, tubulointerstitial nephritis and uveitis syndrome, and inflammatory bowel disease. Less likely malignant etiologies included leukemia, lymphoma and retinoblastoma.
Toxoplasma gondii is a parasitic protozoan thought to be the most common cause of infectious posterior uveitis in the world, infecting about a quarter of the U.S. population and an even greater proportion in other parts of the world.1 Ocular involvement is estimated at 2 percent in the United States. Following initial ocular infection with T. gondii, the recurrence rate is greatest within the following year. Most infections are acquired through ingestion of the oocyte from soil/water or the cyst form from infected meat; only a fraction of cases comprise transplacental transmission.2
We didn’t come across a case report of Toxoplasma chorioretinitis from free-range chickens; however, chickens are considered one of the most important hosts in the epidemiology of T. gondii infection.3 Free-range chickens are a particularly efficient source of infection for cats who then excrete the environmentally resistant oocysts. Additionally, human consumption of undercooked infected chicken meat may play a role.
Toxoplasma chorioretinitis is largely a clinical diagnosis, and while a positive IgG antibody does not confirm the diagnosis, a negative IgM and IgG titer effectively rules it out. Affected individuals often present with unilateral decrease in vision with floaters. Ophthalmic examination typically reveals a posterior or panuveitis, and a subset may have associated elevated IOP.4 The classic retinal findings include a necrotizing chorioretinitis with yellow-white inflammatory lesions with blurred margins and focal vitritis, classically described as a “headlight in the fog.” Our case was different in that there was no old chorioretinal scar. Some cases present with a characteristic lobular perivasculitis, thought to be due to vascular endothelial inflammation.
Treatment of ocular toxoplasmosis remains controversial without a clear evidence-based protocol. Left alone, most cases resolve within two months. However, treatment is typically initiated in cases of vision-threatening lesions of the macula or optic nerve and in immunocompromised patients.5,6 Various combinations of antibiotics and steroids have been studied. There is currently no treatment regimen that definitively improves final visual acuity compared to placebo, but current goals of treatment include shortening the active disease course and reducing recurrence.6 A recent double-masked randomized clinical trial found that intermittent antibiotic treatment with Bactrim decreased the risk of Toxoplasma retinochoroiditis recurrence up to three years following initial infection.7 Additionally, intravitreal injection of clindamycin and dexamethasone may be an acceptable alternative to the classic treatment in ocular toxoplasmosis, as outcomes including visual acuity improvement and vitreous inflammation reduction were comparable to traditional oral agents.8
In conclusion, Toxoplasma chorioretinitis is a clinical diagnosis with characteristic findings, but clinical history does not always include an exposure to cats. Free-range chickens should be considered in cases of Toxoplasma chorioretinitis as a potential vector of disease transmission in cases without feline contact. Treatment is controversial because it hasn’t been shown to alter final visual outcomes, although most clinicians opt to treat severe, vision-threatening cases to shorten the active course and possibly reduce recurrence. REVIEW
1. Holland GN. Ocular toxoplasmosis: A global reassessment. Part I: Epidemiology and course of disease. Am J Ophthalmol 2003;136:6:973-88.
2. Stokkermans TJ, Havens SJ. Toxoplasma Retinochoroiditis. 2018 May 10. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan. Available from http://www.ncbi.nlm.nih.gov/books/NBK493182/.
3. Dubey JP. Toxoplasma gondii infections in chickens (Gallus domesticus): Prevalence, clinical disease, diagnosis and public health significance. Zoonoses Public Health 2010;57:1:60-73.
4. Engelhard SB, Patel V, Reddy AK. Intermediate uveitis, posterior uveitis, and panuveitis in the mid-Atlantic USA. Clin Ophthalmol 2015;9:1549-55.
5. Kartasasmita A, Muntur WP, Enus S, et al. Rapid resolution of toxoplasma chorioretinitis treatment using quadruple therapy. Clin Ophthalmol 2017;11:2133-2137.
6. Pradhan E, Bhandari S, Gilbert RE, et al. Antibiotics versus no treatment for toxoplasma retinochoroiditis. Cochrane Database Syst Rev 2016 May 20;5:CD002218.
7. Fernandes Felix JP, Cavalcanti Lira RP, Cosimo AB, et al. Trimethoprim sulfamethoxazole versus placebo in reducing the risk of toxoplasmic retinochoroiditis recurrences: A three-year follow-up. Am J Ophthalmol 2016;170:176-182.
8. Soheilian M, Ramezani A, Azimzadeh A, et al. Randomized trial of intravitreal clindamycin and dexamethasone versus pyrimethamine, sulfadiazine, and prednisolone in treatment of ocular toxoplasmosis. Ophthalmology 2011;118:1:134-41.



