Retinal arterial macroaneurysms (RAMs) are rare in clinical practice. In this article, we’ll describe the features of RAMs and how to respond if they are identified on clinical examation.
What are RAMs?
The distinct clinical entity of RAM, an aneurysmal alteration of the retinal arteries, has been described in clinical series.1,2 Specifically, RAMs are defined as fusiform or saccular dilatations of the retinal arteries, usually emanating from the first three branches of the arteriolar tree. They typically range from 100 to 250 μm in diameter, commonly involve vessels along the superotemporal or inferotemporal retinal arcades, and often occur at arteriovenous crossings or bifurcation sites. RAMs are seen more often in women than men (ratio of 3:1), typically after 60 years of age, and with a medical history of systemic hypertension and/or arteriosclerotic vascular disease. Studies have shown that 10 percent of patients have bilateral disease and 20 percent have multiple macroaneurysms.1-5 The term “simple RAM” refers to isolated vascular ectasia, while “complex RAM” is used when the ectasia is accompanied by hemorrhage.
Clinical Presentation
Patients with RAM can present with variable symptoms. Often, RAM is noted on clinical examination but the patient is asymptomatic. The involved artery may be narrowed proximally and distally to the macroaneurysm. In other instances, severe vision loss can occur from leakage of the aneurysm with resultant hemorrhage into the vitreous cavity, subhyaloid space and/or intraretinally or subretinally. Many times, “hourglass hemorrhages,” defined as the simultaneous presence of preretinal and subretinal hemorrhage, can be seen. Serous fluid can also collect intraretinally, producing diffuse or focal cystoid macular edema with or without the accumulation of lipid exudates. Secondary epiretinal membrane can form after the resolution of longstanding subhyaloid hemorrhage. In rare cases, large macroaneurysms occurring at an arteriovenous crossing can cause a secondary branch retinal vein occlusion.1-6
RAM Pathogenesis
 |
| Figure 1. Histopathology of a ruptured RAM with preretinal, intraretinal and subretinal hemorrhage. |
The pathogenesis of RAM is thought to be secondary to a combination of several mechanisms causing blood vessel wall weakness and subsequent aneurysmal dilatation. The main mechanisms thought to underlie RAM formation include focal ischemia to blood vessel walls, chronic hypertensive and arteriosclerotic vascular wall damage, and inherent structural defects in blood vessels. Histopathologically, RAMs are found to have arterial dilatation with variable degrees of artery wall hyalinization and surrounding retinal exudate or hemorrhage (Figure 1).7
Imaging of RAM
While the diagnosis of RAM is largely clinical, imaging—especially fluorescein angiography—can be a useful adjunctive tool. On FA, one can see immediate uniform filling of the macroaneurysm (Figure 2). Partial filling may be seen if the aneurysm is spontaneously involuting or is partially thrombosed. Many times, there can be no view of the RAM because it’s hidden by hemorrhage overlying the macroaneurysm. Areas surrounding the RAM can show capillary microaneurysms, nonperfusion, intraretinal microvascular abnormalities and telangiectasis. Leakage can be seen if there is concomitant cystoid macular edema, and distortion of retinal architecture can be seen in the setting of ERM formation. OCT can be used for the identification of subretinal fluid and hemorrhage, macular edema and ERM formation and can be used to monitor the effects of therapy.1-6
Treatment Options
The clinical course of RAMs can be variable. The majority of RAMs can be observed, and many will spontaneously involute. In a natural-history study, Cathleen McCabe, MD, and colleagues described a series of 41 patients with macular hemorrhages secondary to RAMs managed solely with observation. The patients’ average visual acuity at baseline was 20/200 or worse. After an average follow-up of 15.7 months, 37 percent of eyes achieved a final visual acuity of 20/40 or better, 29 percent achieved 20/50 to 20/100, and 34 percent were 20/200 or worse. While good visual acuity outcomes can be achieved with observation alone, poorer visual acuity was associated with macular pigmentary changes after resorption of blood.8
However, intervention may be required in cases of exudative or hemorrhagic RAMs, or recurrent/persistent cystoid macular edema. Laser photocoagulation, first described by J. Donald M. Gass, MD, in 1976, was shown to reduce leakage in approximately 16 to 27 percent of RAMs. Laser of the artery and surrounding area may decrease flow and intraluminal pressure, thereby reducing the macroaneurysm. However, this therapy can be associated with the risk of vascular occlusion, early increase in exudates from selective reabsorption of fluid, arteriovenous shunts, macular pucker and scotomas.5,9 Anti-vascular endothelial growth factor therapy has emerged as a useful treatment modality for RAMs (See table, p. 51). VEGF inhibition is thought to decrease vascular permeability, block angiogenesis to reduce further bleeding and exudation, and decrease the binding of pro-thrombotic VEGF receptor 2.5,10-21 Table 1 summarizes several articles in the literature that analyzed anti-VEGF RAM therapy.
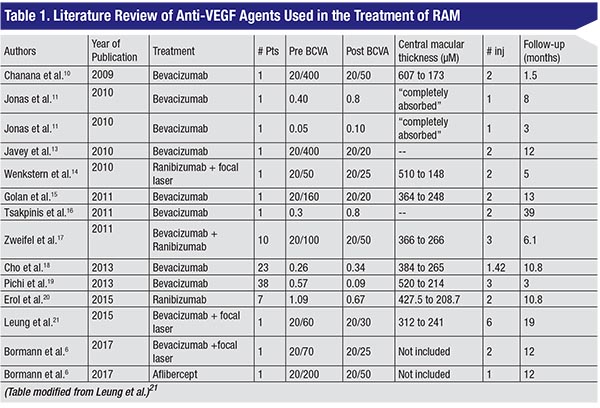 |
In 2009, Delhi, India’s Bhuvan Chanana, MD, and co-workers published the first case report of a patient who received two intravitreal bevacizumab injections (at a concentration of 1.25 mg) for the treatment of cystoid macular edema in the setting of RAM; the patient’s vision improved from 20/400 to 20/50 within six weeks of treatment.10 Han Joo Cho, MD, and colleagues in the department of ophthalmology at the Myung-Gok Eye Research Institute in Seoul, South Korea, described a retrospective interventional case series of 23 patients with symptomatic RAMs who were either treated with intravitreal bevacizumab or observed. While both groups had statistically significant improvements in best-corrected visual acuity and central macular thickness over a 10-month follow-up, the bevacizumab group, which received a mean of 1.4 injections, experienced more rapid improvement.18 Another prospective case series of 38 eyes (19 eyes with hemorrhagic RAM and 15 eyes with exudative RAM) found that that three monthly injections of bevacizumab resulted in improved bestcorrected visual acuity and macular thickness.19 One study of seven patients with either exudative or hemorrhagic RAMs treated with intravitreal ranibizumab reported significant anatomical and visual recovery after 10.8 months, with no associated complications.20 A recent report demonstrated improvement of vision from 20/60 to 20/30 in a patient with a RAM and macular exudation after a series of six injections; however, ultimately focal laser photocoagulation was needed due to recurrence of CME with stabilization of vision at 20/30 at 19 months of follow-up.21 Our case (See Case Report, p. 52) also demonstrates the usefulness of various anti-VEGF agents in the treatment of leakage secondary to a RAM.
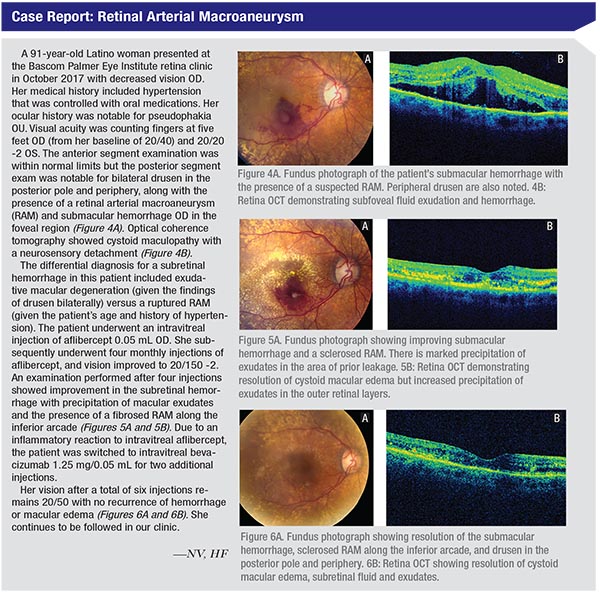 |
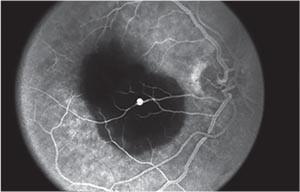 |
| Figure 2. Fluorescein angiography showing pooling within a RAM with blockage from submacular hemorrhage. |
Other treatment options include pneumatic displacement of submacular hemorrhages and pars plana vitrectomy to clear persistent hemorrhages.22,23 In one study from 1998 Mark Humayun, MD, and co-workers described nine eyes with submacular hemorrhage secondary to ruptured RAMs, treated with submacular surgery with tissue plasminogen activator-assisted thrombolysis, with 89 percent of eyes achieving a corrected visual acuity of 20/60 or better.23 These options are currently used less often with thin submacular hemorrhages, given the positive response with anti-VEGF agents.
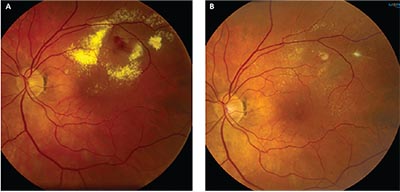 |
| Figure 3A: Fundus photograph showing a leaking RAM and precipitation of exudates in a patient with a visual acuity of 20/60. 3B: Fundus photograph after treatment with both focal laser and anti-VEGF agents, with resolution of hemorrhage and exudates and final visual acuity of 20/40. |
In conclusion, RAMs are rare clinical findings occurring most often in older, hypertensive women. While anti-VEGF therapy can be a useful treatment option to improve vision and decrease macular edema, more complex RAMs may require laser photocoagulation and/or surgical intervention (Figure 3).
Dr. Venkateswaran is a third-year resident at the Bascom Palmer Eye Institute in Miami. Dr. Flynn is a professor at Bascom Palmer and the Donald M. Gass Distinguished Chair in Ophthalmology at The University of Miami, Miller School of Medicine. Dr. Leung is an assistant professor at the Cullen Eye Institute at the Baylor College of Medicine in Houston.
Corresponding Author: Harry W. Flynn Jr. MD, 900 NW 17th Street, Miami, Florida 33136. Email: HFlynn@med.miami.edu.
1. Lewis RA, Norton EW, Gass JD. Acquired arterial macroaneurysms of the retina. Br J Ophthalmol 1976;60:1:21.
2. Robertson DM. Macroaneurysms of the retinal arteries. Trans Am Acad Ophthalmol Otolaryngol 1973;77:OP55.
3. Lavin MJ, Marsh RJ, Peart S, Rehman A. Retinal arterial macroaneurysms: A retrospective study of 40 patients. Br J Ophthalmol 1987;71:11:817-25.
4. Xu L, Wang Y, Jonas JB. Frequency of retinal macroaneurysms in adult Chinese: The Beijing eye study. Br J Ophthalmol 2007;91:6:840–841 5.RabbMF,GalianoDA,TskeMP.Retinalarterialmacroaneurysms. Surv Ophthalmol 1988;33:2:73–96.
6. Bormann C, Heichel J, Hammer U, Habermann A, Hammer T. Intravitreal anti-vascular endothelial growth factor for macular edema due to complex retinal arterial macroaneurysms. Case Rep Ophthalmol 2017;8:1:137-143.
7. Fichte C, Streeten BW, Friedman AH. A histopathologic study of retinal arterial aneurysms. Am J Ophthalmol 1978;85:4:509-18.
8. McCabe CM, Flynn HW Jr, McLean WC et al. Nonsurgical management of macular hemorrhage secondary to retinal artery macroaneurysm. Arch Ophthalmol 2000;118:780-785.
9. Meyer JC, Ahmad BU, Blinder KJ, Shah GK. Laser therapy versus observation for symptomatic retinal artery macroaneurysms. Graefes Arch Clin Exp Ophthalmol 2015;53:4:537-41.
10. Chanana B, Azad RV. Intravitreal bevacizumab for macular edema secondary to retinal arterial macroaneurysm. Eye (Lond) 2009;23:2:493-4.
11. Jonas JB, Schmidbauer M. Intravitreal bevacizumab for retinal macroaneurysm. Acta Ophthalmol 2010;88:7:e284.
12. Jonas JB. Intravitreal bevacizumab for macular complications from RAM. Am J Ophthalmol 2013;155:4:774.
13. Javey G, Moshfeghi AN, Moshfeghi AA. Management of ruptured retinal arterial macroaneurysm with intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging 2010;41:4:1-5.
14. Wenkstern AR, Petersen H. Intravitreal ranibizumab in retinal macroaneurysm. Graefes Arch Clin Exp Ophthalmol 2010;248:11:1667-70.
15. Golan S, Golderberg D, Goldstein M. Long-term follow-up of intravitreal bevacizumab in retinal arterial macroaneurysm: A case report. Case Rep Ophthalmol 2011;2:3:387-91.
16. Tsakpinis D, Nasr MB, Tranos P, Krassas N, Giannopoulos T, Symeonidis C, Dimitrakos SA, Konstas AG. The use of bevacizumab in a multilevel retinal hemorrhage secondary to retinal macroaneurysm: A 39-month follow-up case report. Clin Ophthalmol 2011;5:1475-7.
17. Zweifel SA, Tonz MS, Pfenninger L, Becker M, Michels S. Intravitreal anti-VEGF therapy for retinal macroaneurysm. Klin Monbl Augenheilkd 2013;230:4:392-5.
18. Cho HJ, Rhee TK, Kim HS, Han JI, Lee DW, Cho SW, Kim JW. Intravitreal bevacizumab for symptomatic retinal arterial macroaneurysm. Am J Ophthalmol 2013;155:5:898-904.
19. Pichi F, Morara M, Torrazza C, Manzi G, Alkabes M, Balducci N, Vitale L, Lembo A, Ciardella AP, Nucci P. Intravitreal bevacizumab for macular complications from retinal arterial macroaneurysms. Am J Ophthalmol 2013;155:2:287-294.
20. Erol MK, Dogan B, Coban DT, Toslak D, Cengiz A, Ozel D. Intravitreal ranibizumab therapy for retinal arterial macroaneursym. Int J Clin Exp Med 2015;8:7:11572-8.
21. Leung EH, Reddy AK, Vedula AS, Flynn HW Jr. Serial bevacizumab injections and laser photocoagulation for macular edema associated with a retinal artery macroaneurysm. Clin Ophthalmol 2015;9:601-609.
22. Berrocal MH, Lewis ML, Flynn HW Jr. Variations in the clinical course of submacular hemorrhage. Am J Ophthalmol 1996;122:486.
23. Humayun M, Lewis H, Flynn HW Jr et al. Management of submacular hemorrhage associated with retinal arterial macroaneurysms. Am J Ophthalmol 1998;126:358-361.



