When we’re managing a patient, it’s easy to focus solely on the main problem we’re trying to treat. However, patients often have more than one problem. For example, both
glaucoma and corneal disease are common conditions; about 3.5 per-cent of people in the United States have glaucoma. At the same time, according to the Department of Health and Human Services and the National Keratoconus Foundation, 1 percent have keratoconus, 20 percent have dry-eye disease and 4 percent of patients over age 40 have Fuchs’ dystrophy. With these numbers, it’s inevitable that some patients will have both glaucoma and corneal disease.
Furthermore, some of the treatments we provide to address either problem can create or exacerbate the other. It’s no secret that treatments for glaucoma, whether pharmaceutical or surgical, can have implications for the cornea. For example, about half of glaucoma patients using eye drops develop dry-eye disease. Similarly, treatments for corneal disease (e.g., long-term steroids after full-thickness corneal transplant) can increase the risk of glaucoma. So even if patients don’t have both problems upon presentation, they may have them after our treatment.
This is where MIGS comes into the picture. These minimally invasive procedures give us a way to address (or prevent) elevated intraocular pressure, without using drops that can negatively affect the cornea. At the very least, MIGS can reduce the number of drops patients need to use. For that reason, I believe it behooves us to 1) be aware of the possible negative cycle we may be creating when we treat either glaucoma or corneal problems; and 2) consider the use of MIGS procedures to break up that cycle.
Eye Drops & Corneal Disease
In terms of glaucoma treatment leading to corneal disease, there’s no question that a primary culprit is the use of eye drops to lower IOP. That gives MIGS an advantage. Even if MIGS surgeries don’t result in a complete resolution of elevated IOP in some patients, they almost always reduce the number of eye drops the patient needs to stay in the target pressure zone. This reduction in medication use has been confirmed via a number of studies:
• One recent study involving the iStent Inject found that the mean number of medications needed dropped from 1.6 to 0.4 at 23 months, a 75-percent reduction.1
• Another study found that implanting a Hydrus microstent reduced the mean number of medications from 1.7 to 0.3, an 82.4-percent reduction.2
• A study involving trabeculotomy using the TRAB 360 device found that medication use was reduced from 1.7 to 1.1 at 12 months, a 35-percent reduction.3
• A study combining phaco with a Kahook Dual Blade procedure caused a 70-percent drop in medication use at one year.4
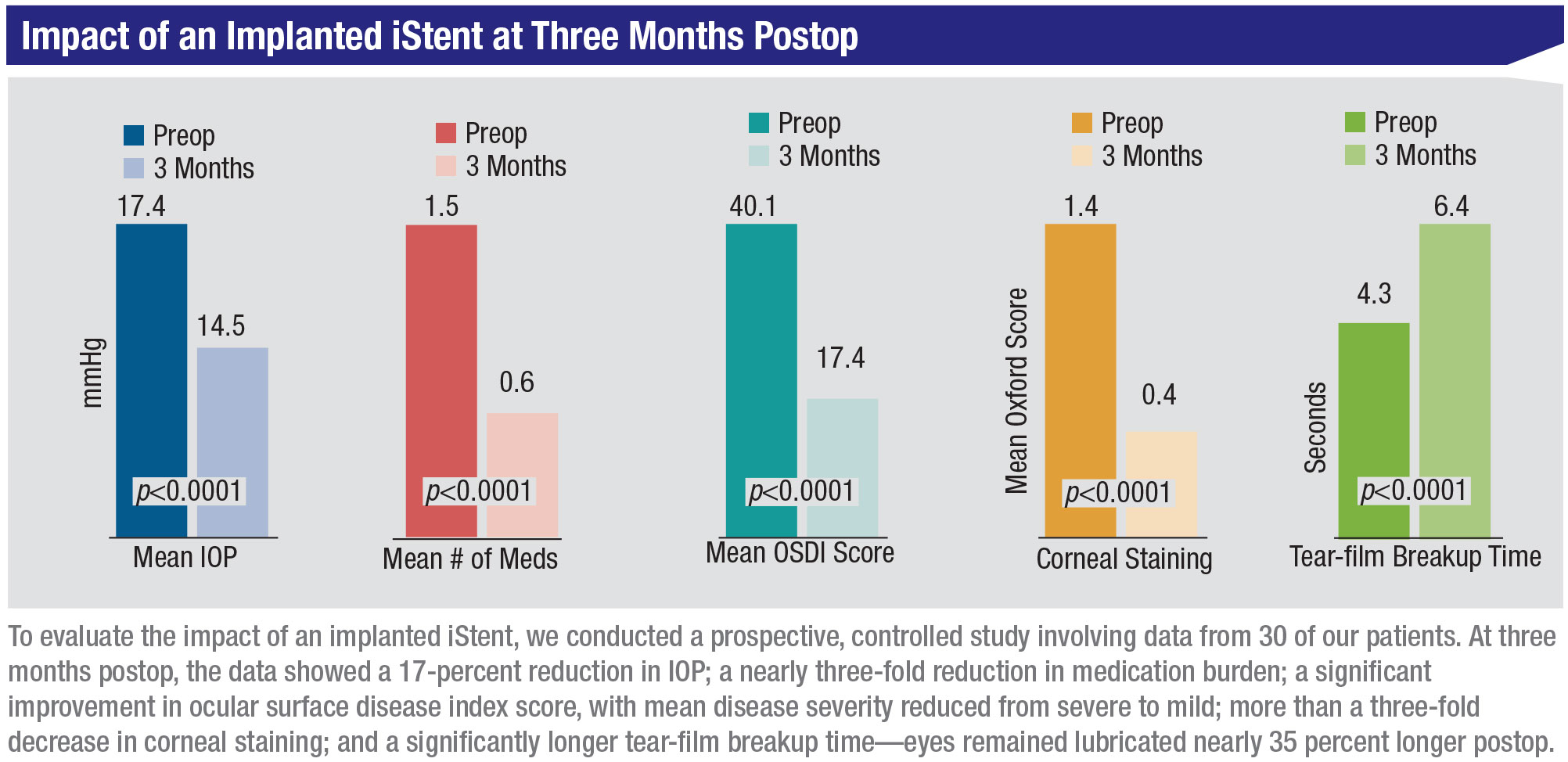
We also conducted a prospective, controlled study in our own practice, evaluating the data from 30 of our patients that had received an iStent. In addition to a mean IOP reduction of 17 percent, medication use dropped by 60 percent, from 1.5 to 0.6 medications. This was accompanied by significant improvements in the corneal surface:
• Ocular surface disease index score in these patients dropped from 40.1 to 17.4 at three months, a 57-percent drop (p<0.0001); average disease severity was reduced from severe to mild.
• The mean Oxford corneal staining score dropped from 1.4 to 0.4 at three months, a reduction of 71 percent (p<0.0001).
• Tear-film breakup time improved from 4.3 seconds to 6.4 seconds at three months (p<0.0001).
The correlation between number of eye drops being used and risk of ocular surface disease has also been confirmed by numerous studies. For example:
• A 2018 study found that amount of meibomian gland loss was significantly correlated with number of medications being used.5 Patients using a greater number of eye drops had significantly shorter tear breakup time (p=0.047), lower meibomian gland density (p=0.032), higher meibomian gland loss ratio (p=0.011) and higher meiboscale (p=0.036).
• A 2017 study found that patients using eye drops had significantly higher OSDI scores (10.24 vs. 2.5; p<0.001) and corneal staining scores (64.93 percent vs. 32.61 percent; p<0.001).6
Given that reality, it should be no surprise that if you can safely de-crease the number of medications by performing MIGS, you’ll improve the condition of the ocular surface.
There’s another aspect to the inter-action between glaucoma and the cornea: Treating one condition can lead to worsening of the other, and vice versa. If our glaucoma treatment causes the patient to develop corneal disease, the temptation may be to address the corneal issues with steroid drops (or a steroid insert). As we all know, steroids can cause elevated IOP, possibly leading to glaucoma. If that occurs, the temptation will be to increase the number of glaucoma drops to lower the pressure, increasing the risk of corneal disease. The result can be an escalating, vicious cycle.
This is another reason to consider making MIGS a part of your armamentarium. MIGS gives us a way to lower both the IOP and the number of medications the patient is using, thus avoiding the vicious cycle that can be triggered by the use of eye drops to lower pressure.
 |
MIGS with Corneal Surgery
Of course, steroids are generally prescribed following corneal surgeries such as penetrating keratoplasty and DMEK, which have also been associated with post-treatment glaucoma. After DMEK, IOP goes up in about 12 percent of patients,7 and we know that about 20 percent of patients develop glaucoma following PK.8 We think that this is primarily a result of the postoperative steroids. DMEK patients have to use steroid drops for a year following the surgery, and patients undergoing a PK sometimes have to use steroids for the rest of their lives. Then, if the steroids trigger elevated IOP, the problem may be compounded by the temptation to treat that with glaucoma drops, potentially injuring an already susceptible cornea. You can end up with the same vicious cycle, with each treatment making the other problem worse.
For this reason, we’ve been investigating the idea of combining MIGS with corneal procedures such as DMEK and PK. Today, this isn’t a common approach. Part of the reason it’s not common may be that many glaucoma surgeons don’t perform corneal surgery, and vice versa. (I’m an exception, having been trained in both cornea and glaucoma.) However, adding MIGS to these procedures can prevent the vicious cycle from starting, leading to better outcomes and happier patients.
We conducted a small study in our practice involving 16 patients with Fuchs’ dystrophy. (We presented some of this data at the 2019 meeting of the American Society of Cataract and Refractive Surgery.) The patients underwent DMEK and a simultaneous trabecular bypass using an iStent. Results included:
• Vision improved; preoperative vision was 20/50, vision at six months postop was 20/25.
• The number of medications remained the same pre- and postop.
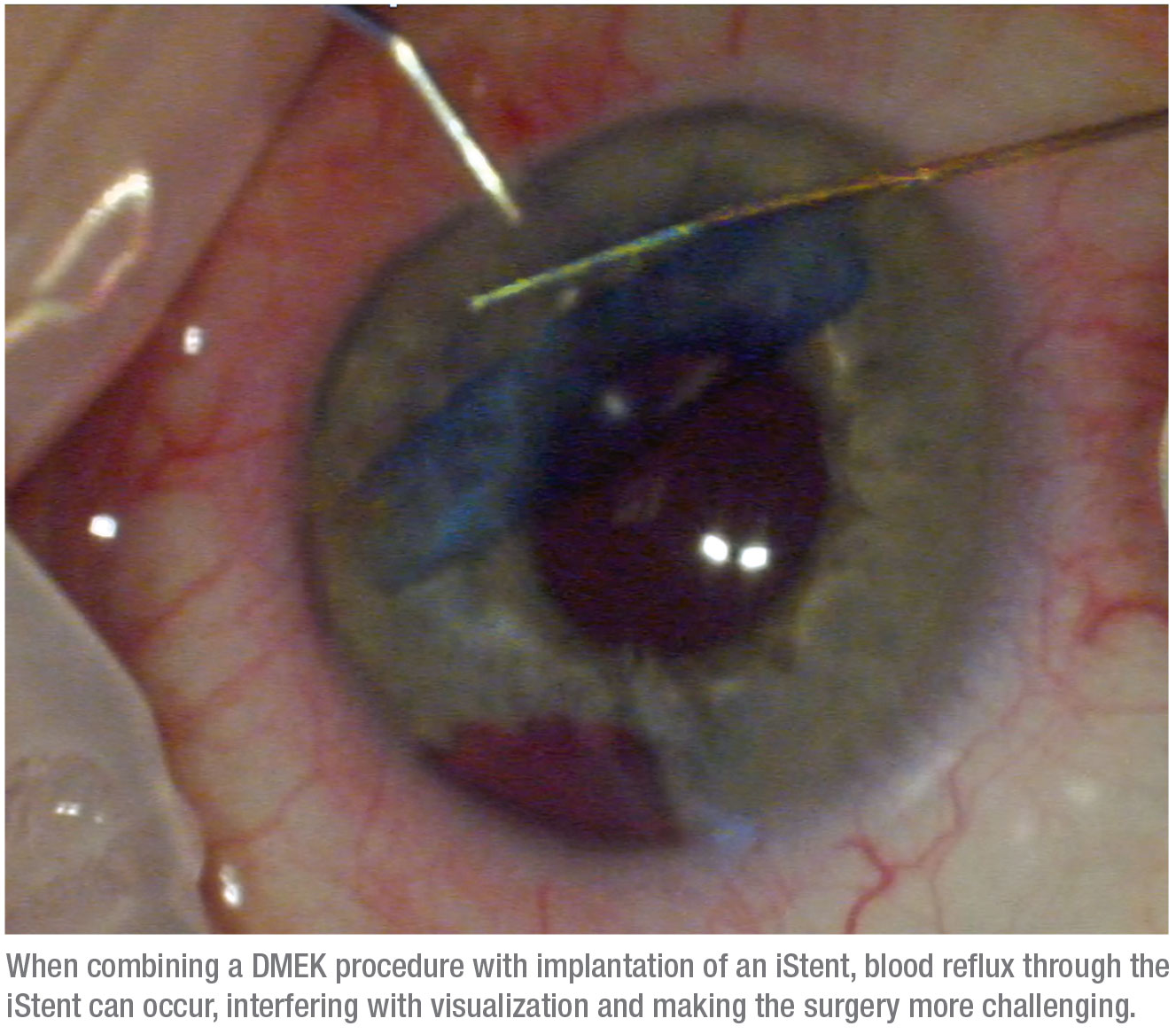
• The re-bubble rate was a little higher than normal at 26 percent. This was probably because of blood reflux, which can be a problem when doing an endothelial cell transplant like DMEK at the same time as a MIGS procedure. When we lower pressure in the anterior chamber, blood reflux can occur, making endothelial cell transplantation more challenging and riskier.
• One of the patients went on to need a tube shunt.
• Most notably, none of the patients’ IOPs increased despite using the post-
operative steroid.
I made a video of one such surgery, in which I combined cataract surgery plus DMEK, and also placed an iStent. (See image, above.) In this case, the lowered IOP that’s part of the DMEK procedure caused blood to reflux through the stent, which might have made it more difficult to unfold the DMEK graft; however, the surgery was successful.
Whether to do the DMEK and iStent procedures separately or concomitantly is an important consideration. I often do them at the same time, but I have mixed feelings about recommending this approach to other surgeons because doing them together can make it very hard to unscroll the DMEK graft.
There’s also the practical issue that in many cases a different surgeon would have to perform each procedure, making it more difficult to combine them in the OR. We’ve occasionally had two surgeons come in for one surgery, but the logistics of that can be challenging. For that reason, if you wish to try combining these surgeries to minimize the patient’s need to use postoperative glaucoma drops, a prudent approach might be to perform the DMEK first and then come back later to do a procedure combining cataract surgery and MIGS, or a standalone MIGS procedure.
I also believe it makes sense to add a MIGS procedure when performing a PK, for the same reason—avoiding the postoperative need for drops that will harm an already fragile cornea. (Corneal surgeons know that many of these patients will develop glaucoma postop.) This is a very controversial topic, because intraoperative surgical complications can occur when MIGS and a PK are performed together. In addition, going back into the eye later, after the patient has a corneal transplant, is not without risk. Nevertheless, if I had my way, I’d do a
MIGS procedure with every corneal transplant to help prevent that vicious postop cycle of cornea and glaucoma treatments from occurring.
 |
Other Alternatives
What about other options for addressing postop pressure elevation when it occurs in corneal surgery patients? Options such as tube shunts can cause corneal decompensation, which would be totally unacceptable in these patients.
Selective laser trabeculoplasty is another option that could prevent postop IOP elevation; the question is whether it would be effective enough to prevent the vicious cycle from starting. In my experience, MIGS procedures—especially a trabecular meshwork bypass procedure like the iStent—are more effective. At a theoretical level, this makes sense: SLT appears to loosen the junctions in the trabecular meshwork, letting more aqueous pass through, but a stent allows fluid to bypass the mesh-work and exit through the stent. So it seems less likely to me that a steroid-induced IOP increase would occur following a stent implantation than following SLT.
Finally, it might soon be possible to address steroid-induced glaucoma by delivering glaucoma drugs intra-ocularly, via an implant, for example, rather than by placing potentially damaging drops on the cornea. This would prevent the cycle of complications from starting, and is an option that should certainly be worth considering.
A New Perspective
I believe it’s important to consider the interaction of corneal and glaucomatous disease whenever we treat glaucoma or corneal issues. Many of our patients present with problems in both areas, and our treatments can trigger—or worsen—either type of problem. Knowing that, we should go out of our way to avoid starting the vicious cycle in which treating one makes the other worse, and vice versa. MIGS procedures give us a way to prevent that from happening, leading to better outcomes, fewer additional drugs and surgeries, and happier patients. REVIEW
Dr. Berdahl is a corneal, refractive and glaucoma surgeon at Vance Thompson Vision in Sioux Falls, South Dakota, and associate clinical professor at the University of South Dakota. He’s consulted for Allergan, Avedro, Bausch + Lomb, Equinox, Glaukos, Imprimis and Neuromedical.
1. Samuelson TW, Sarkisian SR Jr, Lubeck DM, et al, with the iStent inject Study Group. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: Two-year results. Ophthalmology 2019;126:6:811-821.
2. Samuelson TW, Chang DF, Marquis R, et al, with the HORIZON Investigators. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: The HORIZON study. Ophthalmology 2019;126:1:29-37.
3. Sarkisian SR, Mathews B, Ding K, Patel A, Nicek Z. 360º ab-interno trabeculotomy in refractory primary open-angle glaucoma. Clin Ophthalmol 2019;13:161-168.
4. ElMallah MK, Seibold LK, Kahook MY, et al, with the KDB Goniotomy Study Group. 12-Month retrospective comparison of kahook dual blade excisional goniotomy with istent trabecular bypass device implantation in glaucomatous eyes at the time of cataract surgery. Adv Ther 2019;36:9:2515-2527.
5. Cho WH, Lai IC, Fang PC, et al. Meibomian gland performance in glaucomatous patients with long-term instillation of IOP-lowering medications. J Glaucoma 2018;27:2:176-183.
6. Pérez-Bartolomé F, Martínez-de-la-Casa JM, Arriola-Villalobos P, et al. Ocular surface disease in patients under topical treatment for glaucoma. Eur J Ophthalmol 2017;27:6:694-704.
7. Maier AKB, Wolf T, Gundlach E. et al. Intraocular pressure elevation and post-DMEK glaucoma following Descemet membrane endothelial keratoplasty. Graefes Arch Clin Exp Ophthalmol 2014;252:12,1947–54.
8. Kornmann HL, Gedde SJ. Glaucoma management after corneal transplantation surgeries. Curr Opin Ophthalmol 2016;27:2:132–139.



