Nancy M. Holekamp, MD,
Although first described more than 150 years ago, retinal vein occlusion remains controversial in terms of its management. There is currently no medical management strategy proven to be beneficial for RVO. Regarding laser intervention, neither physicians nor their patients are content with the treatment results from the Branch Retinal Vein Occlusion Study and the Central Retinal Vein Occlusion Study. Thus, there is continued investigation into new, alternative surgical treatment strategies for RVO.
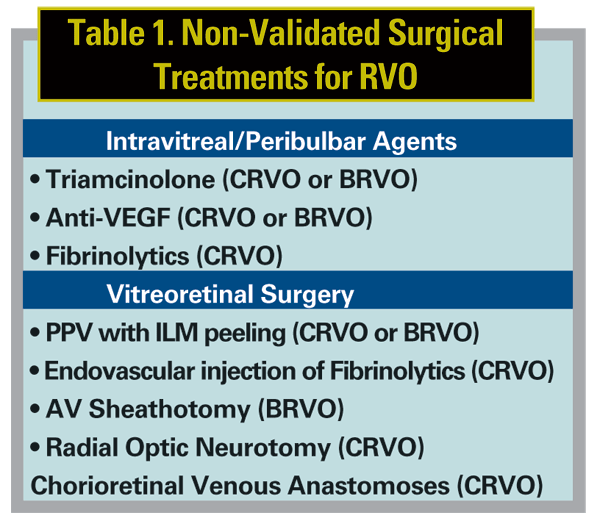
This installment of Retinal Insider will first briefly revisit the classical management of RVO and then provide a detailed overview of new and innovative non-validated surgical approaches (See Table 1). It is important to remember that most of these investigations are far from conclusive due to inadequate study design including non-randomization, lack of controls, insufficient sample size and inadequate follow up.
Classical Management of RVO
The landmark historical studies that defined the current standards of management for vein occlusions are the Branch Vein Occlusion Study (BVOS) and the Central Vein Occlusion Study (CVOS). Both were multicenter, prospective, randomized controlled trials that provided pivotal data evaluating photocoagulation therapy for their respective conditions.
The BVOS demonstrated the efficacy of macular grid laser photocoagulation in patients with chronic (>three months duration) macular edema secondary to BVO resulting in visual acuity of 20/40 or worse.1The study showed a small but significant 1.33 line gain in vision as compared to 0.23 lines in the control group. Their recommendations include observation for three months to allow for resolution of macular edema and hemorrhage.
For patients with 20/40 or worse vision and persistent macular edema, fluorescein angiography should be used to determine the extent of ischemia. In the absence of macular ischemia or foveal hemorrhage as the cause of vision loss, argon laser grid photocoagulation to the area of leakage is recommended by the BVOS.
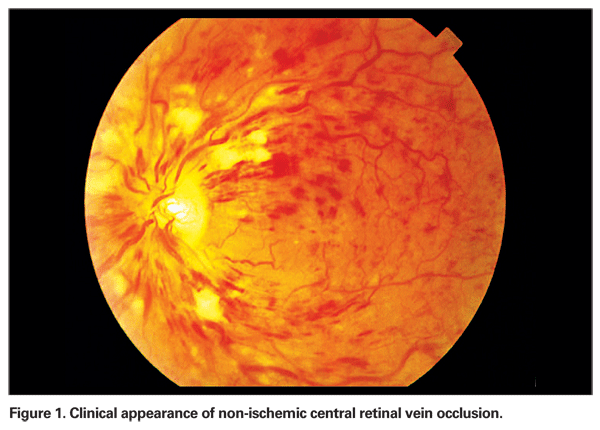
The CVOS compared: 1) the efficacy of immediate prophylactic panretinal photocoagulation in non-perfused CRVO versus observation alone, and 2) the efficacy of macular grid laser photocoagulation in patients with perfused macular edema secondary to CVO resulting in a visual acuity of 20/50 or worse versus observation.2 The study showed no statistically significant differences between treatment and control groups for either of these treatments. They concluded that prophylactic (early) PRP did not prevent iris or angle neovascularization, and recommended that this procedure be performed only when active neovascular disease was demonstrable. They also showed that although grid macular photocoagulation did reduce macular edema, it did not result in an improvement in visual acuity. Thus, no clinically efficacious therapy has yet been proven for macular edema from CRVO (See Figure 1).
Non-Validated Injection Procedures
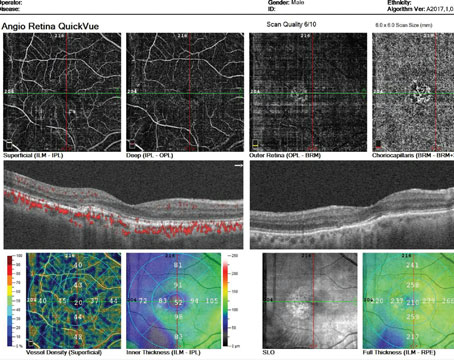
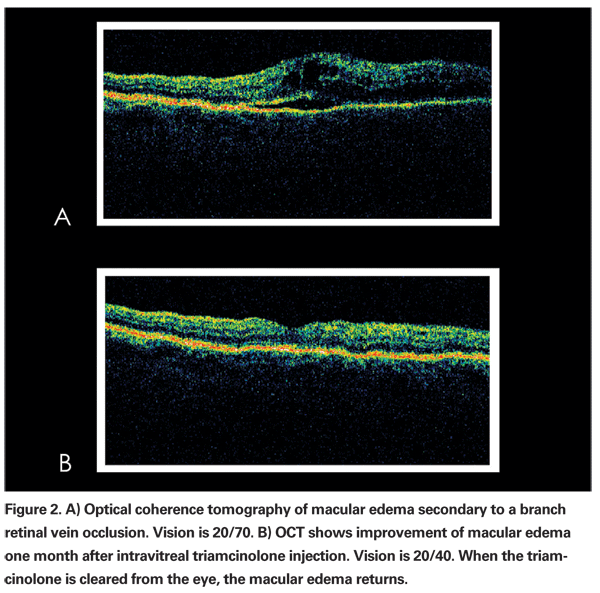
• Corticosteroids. Corticosteroids have been used for the treatment of macular edema secondary to both branch3 and central4,5 retinal vein occlusions. When administered via an intravitreal route, triamcinolone acetonide has been shown to reduce macular edema on optical coherence tomography and improve visual acuity in small, short-term studies (See Figure 2). The Standard Care versus Corticosteroid for Retinal Vein Occlusion Study (SCORE) is an NIH-funded, multicenter, Phase III, randomized, controlled trial comparing efficacy of intravitreal triamcinolone to standard care for macular edema secondary to BRVO and CRVO. Enrollment has been completed with 682 patients, and data should be available in the next several months.
In another Phase III randomized, controlled trial, the biodegradable dexamethasone implant Posurdex has been investigated for the treatment of macular edema in RVO. Enrollment has been completed and results should be released soon. The pharmaceutical company sponsor (Allergan) is anticipating FDA approval. However, because corticosteroids work indirectly by affecting VEGF both at the level of gene expression and through regulation of inflammatory cytokines, any beneficial effect is thought to be temporary. Consequently, repeated doses may be required to prevent recurrence of ME.
Adverse effects of corticosteroids include a significant rise in intraocular pressure, development of cataract and potential risks of endophthalmitis and retinal detachment.
Results from Phase III clinical trials will better define the safety profile of intravitreal corticosteroids.
Anti-VEGF Drugs
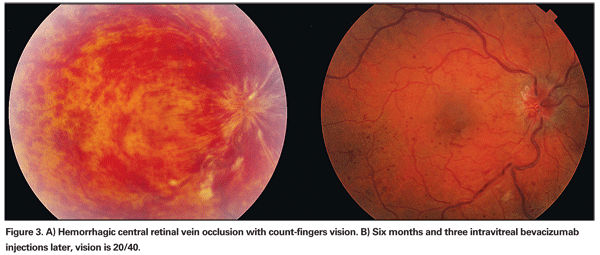
Intravitreal administration of anti-VEGF agents has rapidly been gaining popularity after FDA approval of Lucentis (ranibizumab, intravitreal) for exudative macular degeneration. Ranibizumab and bevacizumab (Avastin) are commercially available recombinant antibodies against all isoforms of VEGF. A handful of small studies, both retrospective and prospective, have shown improvement of macular edema, macular thickness by OCT and visual acuity after multiple administrations of bevacizumab (See Figure 3).6
Variability in frequency, dose and endpoints make these studies difficult to compare; nevertheless, the data appear to be promising.
Two large multicenter, Phase III, randomized, sham-injection controlled studies sponsored by Genentech, are under way to evaluate the efficacy of ranibizumab in acute macular edema for branch (BRAVO Study) and central (CRUISE Study) retinal vein occlusions. Both studies are comparing 0.3 and 0.5 mg ranibizumab versus sham injection at a monthly dosing interval for six months. Primary outcome measures are improvement in best corrected visual acuity and safety profiles. Release of top-line data concerning primary outcome measures is estimated to occur in 2010.
• Fibrinolytics. Intravitreal administration of fibrinolytic agents has also been studied in the management of acute CRVO. The rationale for its use is based on the thrombotic etiology of RVO. Recombinant human tissue type plasminogen activator (tPA) is the preferred agent because of its clot selectivity, superiority to other fibrinolytics in achieving reperfusion and its safety and efficacy in other ocular surgery.7 Several different groups have reported varying degrees of success using intravitreal tPA to achieve reperfusion and visual improvement in CRVO in their small, non-controlled studies. 8,9,10 One of them reported no significant improvement in final visual outcome in their prospective but non-controlled study.9 Dosing of tPA varied between and within studies, and onset of CRVO relative to drug administration was also variable between studies. More recent evidence suggests that the true mechanism by which intravitreal injection of tPA reduces macular edema is the generation of a posterior vitreous detachment, rather than a pharmacologic action of the drug itself.11 As of yet, there is still insufficient evidence to recommend use of intravitreally administered fibrinolytics in CRVO.
Non-Validated Surgical Therapies
Multiple vitreoretinal surgical procedures have been advocated for vein occlusions. These include pars plana vitrectomy (PPV) ±ILM peeling, endovascular tPA, radial optic neurotomy (RON), and chorioretinal venous anastomoses (CVA) for CRVO; and, PPV ±ILM peeling, and AV sheathotomy for BRVO. It should be noted that none have yet been evaluated with a randomized clinical trial, and evidentiary support is based solely on case reports and series.
• Vitrectomy with ILM peeling. Pars plana vitrectomy with or without ILM peeling has been advocated by some for the treatment of persistent macular edema secondary to either type of vein occlusion, especially if associated with the presence of an attached posterior hyaloid face.12-14 The mechanism of action is thought either to be relaxation of vitreomacular traction, increased vitreal oxygenation, reduction in intraocular VEGF or a combination of these factors.
• Retinal endovascular surgery. Early this decade, a technique to deliver very high concentrations of tPA locally to the retinal circulation with minimal concentrations of the drug reaching the systemic circulation was introduced. 15-19 The procedure involves a standard three-port pars plana vitrectomy followed by cannulation of a branch retinal vein with a glass cannula aided by a stabilization system, and injection of 0.6 to 7.5 ml of 200 ug/mL tPA. Early, non-controlled, interventional case series initially showed promise for this procedure with up to 50 to 59 percent gaining ≥3 lines of VA. The purported mechanism by which reperfusion occurs is clot-specific thrombolysis by the fibrinolytic agent, but this has been disputed by others who suggest that this procedure leads to non-specific mechanical dislodging of the thrombus and/or dilation of the central retinal vein. Reported complications include vitreous hemorrhage, cataract, anterior segment and retinal neovascularization, as well as tractional and rhegmatogenous retinal detachments. Technical difficulty, need for specialized equipment and potential surgical complications have limited the widespread use of this technique. Lack of clear efficacy in the absence of concomitantly delivered intravitreal bevacizumab or corticosteroid has led many of the early pioneers of this surgery to abandon it in favor of intravitreal injections alone.
• Radial optic neurotomy. RON is a surgical procedure performed with the goal of releasing a presumed compartment syndrome occurring at the level of the optic nerve and secondarily leading to an increase in venous outflow through the CRV. E. Mitchel Opremcak, MD, and colleagues first described RON in 2001.20 The procedure begins with a three-port pars plana vitrectomy followed by a single incision at the scleral ring with an MVR blade at a clock hour devoid of major blood vessels, cutting an equal portion of the lamina cribrosa and adjacent sclera. A histopathologic study of RON performed on cadaveric eyes suggests that pulsed xenon excimer laser can create a relaxing incision of the cribiform plate and scleral ring similar to the MVR blade incision.21 Although Dr. Opremcak's pilot study, as well as several other small series,22 suggest a notable vision benefit from the procedure, other studies including the Pan-American Collaborative Retina Study Group23 have demonstrated little or no benefit to the procedure with significant complications in >70 percent of study subjects. Skeptics of the procedure question both the existence of a compartment syndrome and the marked potential for vision-threatening complications.24,25 Furthermore, a histopathologic evaluation of an enucleated eye 18 weeks status post-RON revealed scar tissue extending into the lamina cribrosa, and failed to show any histopathologic evidence suggestive of release of a compartment syndrome or increased CRV bloodflow.26
Because vitrectomy surgery increases intraocular oxygenation and thus has a beneficial effect on ischemic retinal disease,27 perhaps vitrectomy alone produced the improvement in vision demonstrated by the earlier studies.
• Chorioretinal anastomosis. Iatro-genically created chorioretinal anastomosis using argon28 or YAG29 laser to rupture Bruch's membrane and the adjacent branch retinal vein is a technique that has also been used to re-establish blood flow through the retinal circulation after RVO. Laser is applied approximately three disk diameters nasal to the optic disk. Multiple treatment sessions may be necessary in order to create a functional anastomosis.30 A modified laser technique in which Bruch's membrane is ruptured but the retinal vessel is left intact has been shown to be safer and more predictable.31
Alternatively a surgical transretinal venipuncture technique can be used to create the anastomosis.32 Anatomical success is determined by ophthalmoscopic appearance as well as retinal angiography.33
The ability to create a successful anastomosis using these procedures is quite variable. The few, small studies thus far reported seem to be promising and show improvement in macular edema and visual acuity. There appears to be a high but poorly defined complication rate that includes intraretinal, subretinal or vitreous hemorrhage, fibro- vascular proliferation and secondary choroidal, retinal or anterior segment neovascularization as well as tractional RD.34
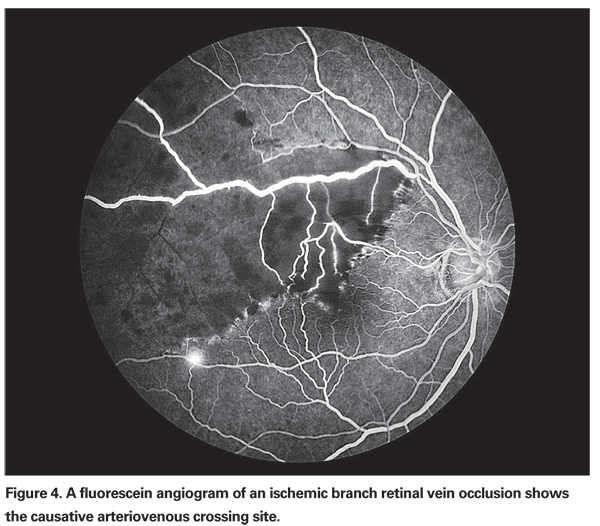
• Branch Retinal Vein Sheathotomy. Since the initial description of surgical decompression of BRVO in 1988,35 arteriovenous crossing sheathotomy has continued to gain popularity. A standard three-port vitrectomy is followed by separation of the artery and vein at the affected crossing site (See Figure 4). Improvement in venous flow is usually noted immediately after the dissection is completed. Multiple retrospective case reports and case series as well as small, prospective, non-controlled studies have shown encouraging results following this procedure.36 Significant improvement in the visual acuity has been demonstrated as early as the second postoperative day.37
Numerous case reports and series have shown resolution of retinal hemorrhages, decreased macular edema, and significant improvement in visual acuity following the procedure. Long-term (five- to seven-year) maintenance of the improved visual acuity has also been shown.38 However, some others have reported minimal or no improvement in vision after the procedure.39,40 Complications include post-vitrectomy nuclear sclerotic cataract, hemorrhage, nerve fiber layer defects and retinal detachment. Though the available literature is promising, larger, randomized, prospective studies are needed to define the role of this procedure in the management of BRVO.
Dr. Ramaiya is in the Department of Ophthalmology and Visual Sciences at Washington University School of Medicine,
1. Argon laser photocoagulation for macular edema in branch vein occlusion. The Branch Vein Occlusion Study Group. Am J Ophthalmol 1984;98:271-82.
2. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology 1995;102:1434-44.
3. Hayashi K, Hayashi H. Intravitreal versus retrobulbar injections of triamcinolone for macular edema associated with branch retinal vein occlusion. Am J Ophthalmol 2005;139:972-82.
4. Ip MS, Gottlieb JL, Kahana A, et al. Intravitreal triamcinolone for the treatment of macular edema associated with central retinal vein occlusion. Arch Ophthalmol 2004;122:1131-6.
5. Park CH, Jaffe GJ, Fekrat S. Intravitreal triamcinolone acetonide in eyes with cystoid macular edema associated with central retinal vein occlusion. Am J Ophthalmol 2003;136:419-25.
6. Badala F. The treatment of branch retinal vein occlusion with bevacizumab. Current Opinion in Ophthalmology 2008;19(3):234-238.
7. Glacet-Bernard A, Kuhn D,
8. Lahey JM, Fong DS, Kearney J. Intravitreal tissue plasminogen activator for acute central retinal vein occlusion. Ophthalmic Surg Lasers 1999;30(6):427-34.
9. Ghazi NG, Noureddine B, Haddad RS, Jurdi FA, Bashshur ZF. Intravitreal tissue plasminogen activator in the management of central retinal vein occlusion. Retina 2003;23:780-4.
10. Elman MJ, Raden RZ, Carrigan A. Intravitreal injection of tissue plasminogen activator for central retinal vein occlusion. Trans Am Ophthalmol Soc 2001;99:219-21;discussion 222-3.
11. Murakami T, Takagi H, Ohashi H, et al. Role of posterior vitreous detachment induced by intravitreal tissue plasminogen activator in macular edema with central retinal vein occlusion. Retina 2007;27:1031-7.
12. Leizaola-Fernandez C, Suarez-Tata L, Quiroz-Mercado H, et al. Vitrectomy with complete posterior hyaloid removal for ischemic central retinal vein occlusion: Series of cases. BMC Ophthalmol 2005;5:10.
13. Mandelcorn MS, Nrusimhadevara RK. Internal limiting membrane peeling for decompression of macular edema in retinal vein occlusion: A report of 14 cases. Retina 2004;24:348-55.
14. Sekiryu T, Yamauchi T, Enaida H, Hara Y, Furuta M. Retina tomography after vitrectomy for macular edema of central retinal vein occlusion. Ophthalmic Surg Lasers 2000;31(3):198-202.
15. Bynoe LA, Hutchins RK, Lazarus HS, Friedberg MA. Retinal endovascular surgery for central retinal vein occlusion: Initial experience of four surgeons. Retina 2005;25:625-32.
16. Weiss JN. Treatment of central retinal vein occlusion by injection of tissue plasminogen activator into a retinal vein. Am J Ophthalmol 1998;126(1):142-4.
17. Weiss JN. Retinal surgery for treatment of central retinal vein occlusion. Ophthalmic Surg Lasers 2000;31(2):162-5.
18. Weiss JN. Retinal endovascular lysis. Ophthalmol-ogy 2008;115:216-7;author reply 218.
19. Weiss JN,
20. Opremcak EM, Bruce RA, Lomeo MD, Ridenour CD, et al. Radial optic neurotomy for central retinal vein occlusion: A retrospective pilot study of 11 consecutive cases. Retina 2001;21:408-15.
21. Soheilian M, Kanavi MR, Rofagha S, Peyman GA. Histopathologic evaluation of optic neurotomy with microvitreoretinal blade or excimer laser in cadaver eyes. Ophthalmic Surg Lasers Imaging 2008;39(1):35-9.
22. Beck AP, Ryan EA, Lou PL, Kroll AJ. Controversies regarding radial optic neurotomy for central retinal vein occlusion. Int Ophthalmol Clin 2005;45(4):153-61.
23. Arevalo JF, Garcia RA, Wu L, et al. Radial optic neurotomy for central retinal vein occlusion: Results of the Pan-American Collaborative Retina Study Group (PACORES). Retina 2008;28:1044-52.
24. Bynoe LA, Opremcak EM, Bruce RA, et al. Radial optic neurotomy for central retinal vein obstruction. Retina 2002;22:379-80;author reply 380-1.
25. Hayreh SS. Radial Optic Neurotomy for Nonische-mic Central Retinal Vein Occlusion. Arch Ophthalmol. 2004;122:1572.
26. Vogel A, Holz FG, Loeffler KU. Histopathologic findings after radial optic neurotomy in central retinal vein occlusion. Am J Ophthalmol 2006;141:203-5.
27. Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens:a possible mechanism for nuclear cataract formation. Am J Ophthalmol 2005;139(2):302-10.
28. McAllister IL, Constable IJ. Laser-induced chorioretinal venous anastomosis for treatment of nonischemic central retinal vein occlusion. Arch Ophthalmol 1995;113:456-62.
29. Quiroz-Mercado H, Sanchez-Buenfil E, Guerrero-Naranjo JL, et al. Successful erbium:YAG laser-in-duced chorioretinal venous anastomosis for the management of ischemic central retinal vein occlusion. A report of two cases. Graefe's Arch Clin Exp Ophthalmol 2001;239(11):872-5.
30. Eckstein M, McAllister I. Laser-induced chorioretinal venous anastomosis for non-ischaemic hemi-central vein occlusion. Clin Experiment Ophthalmol 2000;28(1):18-21.
31. Leonard BC, Coupland SG, Kertes PJ, Bate R. Long-term follow-up of a modified technique for laser-induced chorioretinal venous anastomosis in nonischemic central retinal vein occlusion. Ophthalmology 2003;110:948-54;discussion 955.
32. Fekrat S, de Juan E, Jr. Chorioretinal venous anastomosis for central retinal vein occlusion:transvitreal venipuncture. Ophthalmic Surg Lasers 1999;30(1):52-5.
33. Browning DJ. Fundus photographic, fluorescein angiographic, and indocyanine green angiographic signs in successful laser chorioretinal venous anastomosis for central retinal vein occlusion. Ophthalmol-ogy 1999;106:2261-8.
34. Bavbek T, Yenice O, Toygar O. Problems with at-tempted chorioretinal venous anastomosis by laser for nonischemic CRVO and BRVO. Ophthalmologica 2005;219(5):267-71.
35. Osterloh MD, Charles S. Surgical decompression of branch retinal vein occlusions. Arch Ophthalmol 1988;106:1469-71.
36. Cahill MT, Fekrat S. Arteriovenous sheathotomy for branch retinal vein occlusion. Ophthalmol Clin North Am 2002;15(4):417-23.
37. Wrigstad A, Algvere P. Arteriovenous adventitial sheathotomy for branch retinal vein occlusion:report of a case with longterm follow-up. Acta Ophthalmol Scand 2006;84(5):699-702.
38. Shah GK. Adventitial sheathotomy for treatment of macular edema associated with branch retinal vein occlusion. Curr Opin Ophthalmol 2000;11(3):171-4.
39. Cahill MT,
40. Le Rouic JF, Bejjani RA, Rumen F, et al. Adventitial sheathotomy for decompression of recent onset branch retinal vein occlusion. Graefe's Arch Clin Exp Ophthalmol 2001;239(10):747-51.