When it comes to lowering INTRA- ocular pressure, nothing so far has matched trabeculectomy with mitomycin-C; the efficacy of this procedure has dramatically increased the likelihood of surgical success. In fact, it may be the only procedure where we occasionally see patients who end up with pressures that are too low.
Mitomycin-C is an antifibrotic agent, not a true antimetabolite like the anti-scarring agent 5-fluorouracil. 5-FU appeared in the 1980s and initially showed promise by producing an improved success rate in glaucoma filtering surgery. However, it has several downsides: It has to be injected into the eye once a day (sometimes twice) for one to two weeks; and it can lead to corneal epithelial loss, wound leaks and toxicity. Perhaps more serious, the advantages associated with its use diminish significantly after about six months.
Mitomycin-C is a different story. It's at least 100 times more potent than 5-FU and much longer-lasting. The reason for this is its method of action: It initiates apoptosis, or programmed cell death. In essence, after exposure to mitomycin-C, the DNA in the cell nucleus is reprogrammed, telling the cell to shut itself down and die. Most important, cells can and do transmit that information to other cells that were never in direct contact with the drug. 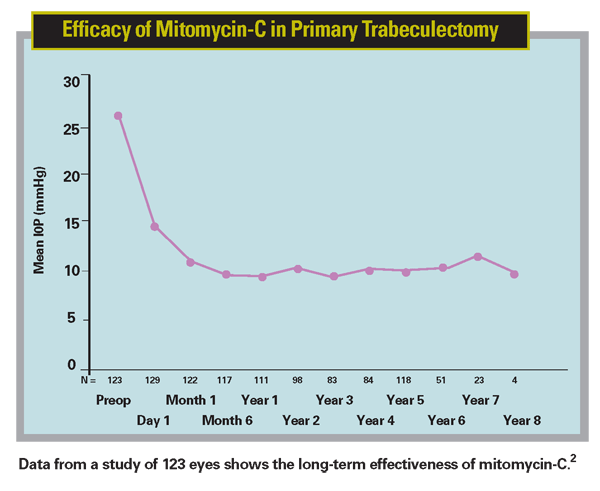
The result is the creation of a sort of apoptotic killing field. Unfortunately, once started, we can't control the apoptosis; every year the area where the surgery was done may look different. And that's the big problem with mitomycin-C: Because the apoptosis can be passed from cell to cell indefinitely, the effect can gradually increase, even years after treatment. (See chart, Hypotony Incidence.) Without warning, patients can develop hypotony because the healing process becomes less effective. Even worse, they can develop blebitis, with potentially devastating consequences.
Trying to Control the Effect
The biggest problem with this drug is that we don't know how to control it. The individual response to mitomycin-C is highly variable, making it impossible to predict exactly what the effect will be. We can't say that if we only expose a patient for one minute her pressure won't go below 10 mmHg. We do know that some patients are at greater risk of developing low pressure late after surgery—such as young, nearsighted females—but late-term hypotony can happen to any patient.
Once cells have been exposed to mitomycin-C, the effect seems impossible to turn off, except by removing the cells carrying the self-destruct message. In one unpublished study Drs. Griffith Steiner, Phillip Campbell and I cultured human Tenon's capsule fibroblasts and exposed them to mitomycin-C, hoping that we could find some way to reverse its effect. We created "wounds" in the cultured cells and monitored them. Control cells that had no mitomycin-C exposure closed the wounds within 24 to 36 hours. In contrast, the cells exposed to mitomycin-C initially exhibited the natural response to a wound, migrating and proliferating to a small degree, but within 12 hours or so retracted and showed a loss of cellularity. None of the wounds in those cell groups ever closed. (See chart, Wound Closure in Cultured Tenon's Capsular Fibrosis.)
To counteract the effect, we tried bathing the exposed cells in fibroblast growth factors, very strong messengers that normally cause fibroblasts to proliferate. This had no effect. In fact, nothing we tried was able to stimulate the cells to grow once they were exposed to mitomycin-C. Other researchers have found the same thing.1
We did find that we could get the wounds to close by injecting or infusing a fresh batch of cultured fibroblasts that had never been exposed to the drug. This is akin to what we have found in the real-life situation in which we advance conjunctival cells into the area where there has been loss of the healing response, in order to produce some healing. The downside of this approach is that we still can't control the result. When we studied the results of conjunctivaplasty on 53 eyes, used to reverse mitomycin-C-induced hypotony, about 50 percent eventually needed glaucoma medications because their pressures ended up too high again. And 10 or 20 percent eventually needed more glaucoma surgery (usually a tube shunt this time around). The good news is that we were successful at reversing the hypotony in most patients.
Autologous blood injections into the filtering bleb have also been tried as a treatment for mitomycin-induced hypotony. Unfortunately, our attempts to achieve success using this approach have so far produced poor results.
Managing Late-Term Hypotony
The hypotony sometimes observed after using mitomycin-C has introduced a new wrinkle to trabeculectomy. Before mitomycin we often saw hypotony and low pressures in the early postop period when an eye hadn't done enough healing. Now, for the first time, patients that we consider great success stories are returning months or years later with their pressure down to zero. This kind of late hypotony is unprecedented.
As someone doing a lot of glaucoma surgery with mitomycin-C, it became clear to me early on that this new hypotony problem would not be easy to fix. To help shed more light on this, Dr. Rajiv Bindlish and I looked at 123 eyes of primary glaucoma surgery patients that we were able to monitor for a minimum of five years. We found that mitomycin-C was effective in producing low long-term IOPs. However, beyond six months post-surgery about 40 percent of the patients exhibited pressures at some time point that we would consider hypotonous.2
Of course, not every patient who comes in with hypotony needs treatment. Some patients can tolerate hypotony—particularly older patients, who tend to have more rigid scleras. That rigidity allows them to maintain good vision for years, even with pressures between 0 and 2 mmHg. However, even in these patients the low pressure can lead to a breakdown in the corneal endothelium—hypotony keratopathy—after many years; the eye may develop a thick and wrinkled cornea. In the meantime, younger and usually nearsighted patients don't usually tolerate low IOPs well at all.
Ironically, it's hard to know exactly what constitutes hypotony. Most ophthalmologists accept the dictum that when a patient is at risk, IOP should be lowered to 12 mmHg or less. However, one of my patients had a pressure close to 12 mmHg and began to develop the signs of hypotony maculopathy—wrinkling of the retina and blurring of the vision.
Until recently, this phenomenon was very uncommon in glaucoma surgery over the long term unless there was a leak or some other process at work. Now I see this condition more frequently, particularly in young, female, nearsighted patients. Just as we can have low-tension glaucoma, perhaps we can have high-tension hypotony.
Fortunately, when I re-operated and brought this patient's pressure back up, her vision improved and she's done well. She's on medication, and her pressures may not be as low as we'd like, but she functions better and she's happier with her vision now than when her pressure was too low.
Treating Hypotony
When a patient returns to your practice with hypotony a year or more after successful trabeculectomy involving mitomycin-C, consider the patient's situation carefully. If the patient's IOP hasn't been low previously, but now the bleb is leaking and not responding to other treatment, a conjunctival graft to resurface the bleb may be the best alternative. If the bleb isn't leaking but is filtering too well, you may need to reinforce the scleral opening with a patch or graft, or resuture the old flap (if possible), in addition to bringing in new conjunctival tissue.
In some situations I simply shuck out the old bleb like an oyster and rely on transplanted conjunctival fibroblasts to create enough scar tissue to raise the pressure. (If the conjunctiva is not too thin, and the aqueous is overflowing at the level of the sclera, then I might choose to skip the conjunctival graft and instead make an incision going under the original bleb so I can close up the sclera.)
Of course, if the transplanted fibroblasts end up doing their job too well, the patient's pressure could go back up to a dangerous level again. In actuality, the propensity of mitomycin-C-exposed cells to pass along their apoptotic message seems to prevent that from happening in many cases, even when I've brought in new conjunctival tissue. The earlier mitomycin exposure still has a tempering effect, preventing the new tissue from dramatically over-healing. Nevertheless, as noted earlier, many patients will overshoot the ideal IOP, ending up with a higher pressure than we'd like; they may eventually need to use drops or have further surgery.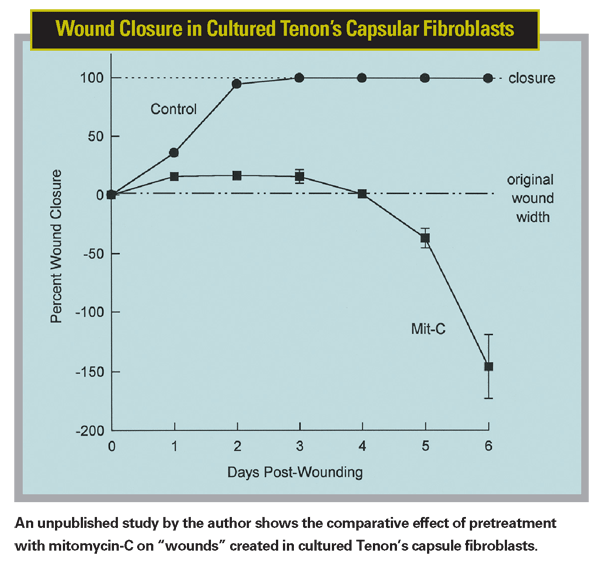
Which course of action is best depends on the presentation and the morphology of the bleb as well as on which factors are responsible.
Managing Late-Term Blebitis
Using mitomycin-C also creates the potential for a late bleb infection that can occur years after the surgery, potentially leading to blindness—and the more successful the surgery, the more we need to be concerned about this possibility. Fortunately, fourth-generation fluoroquinolones such as moxifloxacin and gatifloxacin have given us better treatments for the culprits that usually cause late bleb infections: streptococcus and Haemophilus influenzae.
However, even with these weapons in our armamentarium, catching an infection quickly is crucial. Surface treatment with drops can be quite effective if you catch the blebitis before the infection is really full-blown. So, being proactive can make all the difference.
If I have a patient who has a thin filtering bleb that looks like it could be predisposed to infection, I educate the patient thoroughly about the early symptoms of a bleb infection—i.e., foreign body sensation, pain, reduced vision and light sensitivity—and I have the patient carry an unopened bottle of one of these drugs with him so it will always be within 20 minutes' reach. This makes it possible for the patient to initiate therapy as soon as he has any of the early symptoms. I explain that it's like carrying nitroglycerine or an inhaler or a fire extinguisher; if you sense trouble, don't wait until someone from the office calls you back or a doctor sees you to start treatment.
In my practice, this strategy has been very beneficial. On numerous occasions patients have showed up in my office a day or two after first noticing symptoms and starting treatment, and I find they have a bleb-related infection that's already starting to resolve. Especially with these newer meds, our success at preventing infection from expanding and getting into the eye has been remarkable.
Minimizing Future Problems
In my view, a key part of performing this surgery is minimizing future problems by doing the best possible surgery to begin with. In this respect, two strategies have served me well.
First, when using mitomycin-C, I err on the side of caution. So far, no one has established convincing guidelines for titrating the use of mitomycin-C; in fact, we don't even know whether controlling the dose will control the response. (If we could be sure of the outcome, every cataract surgeon would be performing trabeculectomy. Instead, most send these patients to a glaucoma specialist.)
Nevertheless, being uncertain of a dose-response curve doesn't mean we should overtreat—especially knowing that serious consequences can follow years later. Surgeons currently use a wide range of concentrations and exposure times. In the tube vs. trabeculectomy study, for example, they exposed every eye to 0.4 mg/cc of mitomycin for four minutes.3 The concentration I use varies from 0.2 mg/cc to 0.5 mg/cc, depending on the circumstances: Is there a lot of scar tissue? Is this the second operation? Are there a lot of factors that might predispose the eye to failure? My exposure time is usually around one minute—rarely more than that.
I would urge glaucoma surgeons to err on the side of using less-concentrated solutions and shorter exposure times. Given the choice of operating on a patient whose pressure was still too high or had fallen too low, I'd choose the former, as I suspect most glaucoma surgeons would.
The second suggestion I would offer is using fornix-based flaps rather than limbal-based flaps. Making this switch changes bleb morphology; the more posterior exposure to mitomycin-C promotes a more diffuse filtering bleb. However, this change is only helpful if you have an effective, watertight closure of the conjunctiva incision. Otherwise, all bets are off.
The Best We've Got, for Now
It's interesting that despite the significant dangers connected to the use of mitomycin-C we continue to use it. It seems that the immediate positive result and general long-term success of the surgery is appealing enough to override our knowledge that trouble can occur down the road. Of course, the other key factor is that the demons are treatable; we do have ways of managing late-term bleb malfunctions.
In truth, the majority of patients undergoing this surgery do remarkably well. It's the one surgical technique that often gets patients off of glaucoma drops, for which many patients are tremendously grateful. And, it doesn't preclude the possibility of undergoing cataract surgery sometime in the future. Of course, there's tremendous interest in trying to find something better, something more predictable. But for now, this is the surgery that works the best in most surgeons' hands.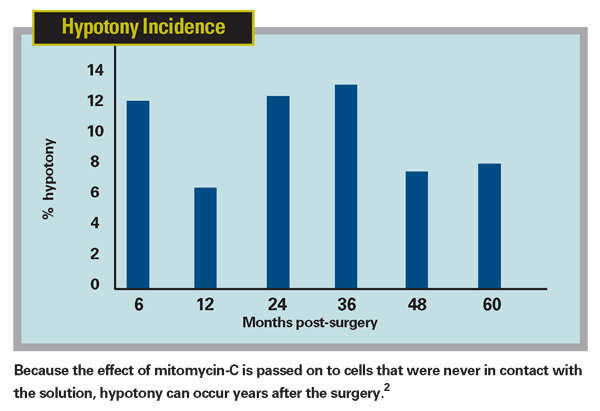
Hopefully, a better surgical alternative will eventually emerge. In the meantime, I advise people who are just embarking on doing trabeculectomy with mitomycin-C to make sure they understand the problems that can occur late-term. You shouldn't just pull the trigger and walk away, leaving someone else to deal with the fallout. And, I advise them to pay attention to intraoperative detail. If you perform meticulous surgery, your outcomes will be more predictable, and both you and your patients will end up happier.
Dr. Condon is associate professor of ophthalmology at Drexel University College of Medicine in
1. Kim, Kim, Song et al. Mitomycin-C induced apoptosis in cultured human Tenon's fibroblasts. Korean J Ophthalmol 1999 13:1:7-15.
2. Bindlish R, Condon GP et al. Efficacy and safety of mitomycin-C in primary trabeculectomy: Five-year follow-up. Ophthalmology 2002;109;7:1336-41.
3. Gedde SJ, et al; Tube Versus Trabeculectomy Study Group. The tube versus trabeculectomy study: Design and baseline characteristics of study patients. Am J Ophthalmol 2005;140:2:275-87.



