Over the past five years, retina practices have changed dramatically, in large part due to the commercial availability and utilization of anti-VEGF, or vascular endothelial growth factor, agents for the treatment of neovascular age-related macular degeneration and retinal vascular diseases. Intravitreal injections have become a routine part of all practices, with most retinal specialists performing multiple intraocular injections on any given clinic day.
While the widespread use of intravitreal VEGF inhibitors has undoubtedly provided tremendous benefit, and the intraocular delivery of these agents is generally well-tolerated by the vast majority of patients, local complications can occur following intravitreal injection, including injection-related inflammation; traumatic injury to the lens capsule; retinal detachment; vitreous hemorrhage; and endophthalmitis.1-3 In addition, tears or rips in the retinal pigment epithelium have been observed following IVT injection, leading some to suggest that either the VEGF inhibitors, the injections themselves or both may promote such events. This update focuses on the two complications of IVT injection with the greatest risk of producing severe and permanent vision loss—acute endoph-thalmitis and RPE tears.
 Endophthalmitis
Endophthalmitis
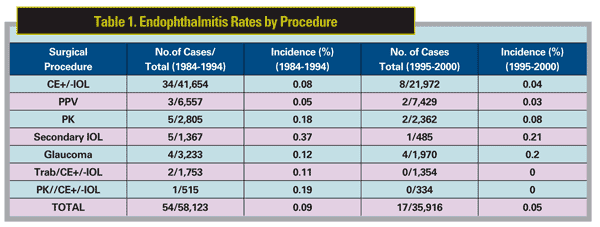
Acute endophthalmitis is perhaps the most feared complication of any penetrating ocular procedure. The incidence of infection following IVT injection is low, and is generally taken to be similar to that reported for other intraocular procedures, or about 0.05 percent, which represents a risk of endophthalmitis occurring once in every 2,000 injections (Table 1).1-4 Given the high number of injections that are performed, however, the absolute number of even low incident events, such as acute endophthalmitis, can be expected to increase dramatically. In 1998, for example, approximately 4,000 intravitreal injections were billed to Medicare. This is in contrast to 2008, when more than 1,000,000 were billed—a figure that has since probably doubled (personal communication, George Williams, MD; See Figure 1). Naturally, such a dramatic rise in the number of IVT injections would be expected to translate into a similar increase in the number of injection-related complications. Moreover, many patients require multiple injections over time, proportionately increasing their cumulative risk. The threshold for suspecting acute endophthalmitis following IVT injection should, therefore, be low.
While pseudoendophthalmitis of the type seen following injection of triam- cinolone acetonide5-7 (See Figure 2) has not been reported with anti-VEGF agents, acute non-infectious inflammation has been reported following injection of bevacizumab and ranibizumab and tends to have a shorter time from injection to onset of symptoms and a milder overall presentation than does post-injection endophthalmitis.8,9 Most recently, Daphna Mezad-Koursh, MD, and associates reported an average of one vs. three days to presentation and better vision, less pain and less vitreous and anterior chamber inflammation (including a notable lack of fibrin, posterior synechiae formation or hypopyon) in patients with non-infectious vs. infectious inflammation following injection of bevacizumab or ranibizumab.9
Data concerning the incidence of infection following intraocular injection comes from both prospective and retrospective studies—including studies that occurred both before and after the advent of anti-VEGF agents. Rama D. Jager, MD, and colleagues performed a comprehensive review of studies published prior to 2004, representing approximately 15,000 injections.1 Therapeutic agents analyzed in their review included triamcinolone acetonide, antiviral agents, intravitreal gases, and tissue plasminogen activator. The overall rate of endophthalmitis calculated in the study was 0.3 percent per injection. However, when cases of presumed sterile endophthalmitis following triamcinolone acetonide injection were removed, the rate was reduced to 0.2 percent per injection.

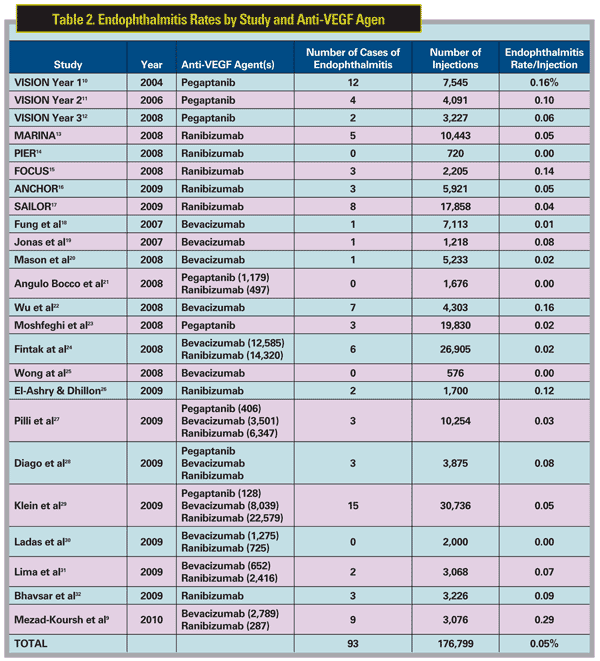
Pegaptanib sodium (Macugen) was the first anti-VEGF drug to be approved by the Food and Drug Administration for the treatment of neovascular AMD in December 2004. During the first year of the Phase III trials of pegaptanib (the VISION trials), the incidence of endophthalmitis was similar10 to that reported in the review by Dr. Jager and colleagues.1 Specifically, the VISION trials reported 12 cases of endophthalmitis following 7,545 IVT injections performed on 892 patients, or a per-injection rate of 0.16 percent. Of note, however, protocol violations occurred in 75 percent of injections associated with acute endophthalmitis, including, most commonly, failure to use a lid speculum and the practice of performing a prophylactic anterior chamber paracentesis to minimize the risk of post-injection pressure spikes. Stricter adherence to the protocol following the first year of the trial resulted in an acute endophthalmitis rate of 0.1 percent (four or 4,091 injections) in year two of the study,11 and 0.06 percent (two of 3,227 injections) in year three of the trial (Table 2).12 The question remained, however: How frequently would acute endophthalmitis occur following IVT injection in subsequent studies and, more importantly, in clinical practice?
 The rates of endophthalmitis reported with ranibizumab (Lucentis) have compared favorably with year three of the VISION trials. To date, safety data have been reported for a total of 37,147 injections performed in five trials, including
The rates of endophthalmitis reported with ranibizumab (Lucentis) have compared favorably with year three of the VISION trials. To date, safety data have been reported for a total of 37,147 injections performed in five trials, including
Endophthalmitis rates from clinic-based series with anti-VEGF agents have generally supported these results. To date, 16 clinic-based studies have included a total 124,789 intravitreal injections of various anti-VEGF agents, reporting a total of 56 cases of endophthalmitis for an overall per injection rate of endophthalmitis of 0.04 percent.9,18-32 Taken collectively, the overall per-injection rate of endophthalmitis for the anti-VEGF agents since their introduction appears, therefore, to be about 84 per 176,799, or 0.05 percent—a number that is virtually identical to the risk for eye surgery in general (Tables 1 & 2).
Given this risk of severe vision loss that accompanies each case of endophthalmitis, however, how can clinicians minimize the risk of infection following any given intravitreal injection? In 2004, an expert roundtable was held to discuss best practice guidelines intended to minimize the incidence of endophthalmitis following IVT injection.33 Protocols from various studies were reviewed, including that used in the VISION trials. Requirements varied greatly regarding the injection procedure, utilization of antibiotics and the need for follow-up. Attempts at forming a consensus opinion were made, and evidence-based recommendations were provided, when possible. Disagreement existed in many instances, as there was little scientific evidence to support particular practice patterns. The group generally recommended the following practices:

Consensus could not be reached on several issues. These included:
- need for a povidine flush of the ocular surface and fornices;
- use of a sterile drape (most did not);
- use of gloves (most did);
- use of pre- or post-injection topical antibiotics; and
- need for follow-up examination versus telephone contact.
RPE Tears
Tears of the RPE are seen in 2 to 3 percent of eyes with neovascular AMD, and perhaps in as much as 10 to 15 percent of eyes with vascularized pigment epithelial detachments (PED).36,37 While certainly not a new clinical entity, RPE tears have been recognized and well described for nearly three decades. RPE tears as a spontaneous development in eyes with neovascualar AMD were described in 1981 and the authors noted the occurrence in association with a PED.38 Since that initial description, RPE tears have been further characterized, and we now recognize several hallmark features in these lesions.39-45 Clinically, patients with an RPE tear may either be asymptomatic or come to attention because of an acute, marked decline in visual acuity. Ophthalmoscopically, RPE tears are characterized by a well-defined area of bared choroid that appears hypopigmented relative to the adjacent area of hyperpigmented scrolled RPE. (See Figure 3A). Imaging with fluorescein angiography reveals a sharply demarcated window defect corresponding to the area of absent RPE, adjacent to an area of blocked fluorescence produced by the scrolled RPE. (See Figure 3B,C). OCT imaging may demonstrate focal loss of the RPE layer in the area of bared choroid, adjacent to a dome like, hyperreflective structure corresponding to the scrolled RPE. The vast majority of RPE tears have a pre-existing vascularized PED,36,37,46,47 and the most important predisposing risk factor appears to be PED size as measured by basal diameter37 and vertical height.37,47
 In addition to occurring spontenously,36 reports exists of RPE tears following conventional laser,48,49 photodynamic therapy,50-55 corticosteroid injections,56,57 and IVT injection of the anti-VEGF agents, including pegaptanib,57-59 ranibizumab,36,60-65 and bevacizumab,15,25,37,66-78 have appeared in the literature. Unfortunately, none of the primary anti-VEGF trial reports mentioned the rate of RPE tears in the various treatment arms,10-17 and reports of incidence in clinic-based surveys have been relatively infrequent and generally fail to provide per injection event rates.
In addition to occurring spontenously,36 reports exists of RPE tears following conventional laser,48,49 photodynamic therapy,50-55 corticosteroid injections,56,57 and IVT injection of the anti-VEGF agents, including pegaptanib,57-59 ranibizumab,36,60-65 and bevacizumab,15,25,37,66-78 have appeared in the literature. Unfortunately, none of the primary anti-VEGF trial reports mentioned the rate of RPE tears in the various treatment arms,10-17 and reports of incidence in clinic-based surveys have been relatively infrequent and generally fail to provide per injection event rates.
Anne E. Fung, MD, and colleagues provided the first such estimate from their Internet-based survey of 70 centers in 12 countries, where they reported four RPE tears following 7,113 injections of given to 5,228 patients, or a per injection rate of 0.06 percent.18 The number of eyes, as well as information regarding timing of the tears to the injections and the nature of the lesions, including specific mention of whether there was a pre-existing PED was, not reported. Since that first report, several clinic-based studies have provided estimates of RPE tear incidence in eyes treated with anti-VEGF agents. Andreas Weinberger and associates observed four RPE tears in 31 eyes (12.9 percent) with neovascular AMD and PEDs, each of which occurred following the first injection.76 Mean vision improved by nine letters at three months in the eyes with the tears with continued bevacizumab treatment. Researchers at Stanford University observed five tears in 173 eyes of 158 patients with neovascular AMD treated with bevacizumab, a per eye rate of 2.9 percent.25 This cohort included approximately 70 patients with a pre-existing PED, 7 percent of whom developed a tear. Bradley Smith, MD, and associates reported a single RPE tear following a first injection in one of 666 ranibizumab injections given to 164 consecutive patients, yielding a 0.61 percent per-patient rate and a 0.15 percent per-injection rate.63 This patient had a vascularized PED with a greatest linear diameter of just over 3 mm. Among eyes with vascularized PEDs at baseline, the per-injection rate was 0.95 percent.
In 2007, Clement Chan, MD, and associates examined 1,280 eyes of 1,255 patients seen at seven centers who received 2,890 injections of bevacizumab.77 A total of 21 eyes in 1,010 evaluable patients developed RPE tears for a per-eye tear rate of 2.1 percent and a per-injection tear rate of 0.73 percent. All tears occurred in eyes with a pre-existing vascularized PED, of which there were 125, resulting in a per-eye tear rate in this subgroup of 16.8 percent. The mean time from injection to RPE tear was about one month, but ranged from four days to about three months. The authors observed no statistically significant difference in pre- and post-RPE tear best correction vision, speculating that this was most likely due to continued CNV suppression provided by ongoing anti-VEGF therapy. The mean time from bevacizumab injection to RPE tear was approximately four weeks.
Lazaros Konstantinidis, MD, and colleagues observed four RPE tears in 72 consecutive patients (5.6 percent) with predominantly classic CNV and in seven eyes of 55 patients (12.3 percent) with PED and occult CNV, all of whom were treated with ranibizumab.64 The mean time to development of a tear in patients with classic CNV was 4.25 months following a mean of four injections, whereas a mean of 1.8 injections was given prior to development of a tear in those patients with a PED and occult CNV. Both patient groups showed improved best-corrected vision post-injection with continued ranibizumab therapy despite the occurrence of a tear. Iris Moroz, MD, and associates studied optical coherence tomography predictors of ROE tears following bevacizumab injection in 143 consecutive patients with neovascular AMD, including 24 eyes with a serous PED.78 Six eyes developed an RPE tear following the first injection, each of which had a pre-existing PED (4.2 percent overall; 25 percent among PED). Each of the six patients who developed a tear showed either a "wavy" appearance with small interruptions or a step-like break or interruption in the continuity of the RPE layer (See Figure 4).37,78
 A 2010 report by Dr. Chan and colleauges37 observed 22 RPE tears in a cohort of 1,002 evaluable eyes (2.2 percent) with neovascular AMD treated with bevacizumab, all of which occurred in the subgroup of 123 eyes with a pre-existing PED (17.9 percent). There was no statistically significant difference in the pre- and post-treatment vision in these 22 eyes despite the fact that only six (27.3 percent) continued to received anti-VEGF therapy. Recently, Dr. Cunning-ham and colleagues79 examined the incidence of RPE tears in the ANCHOR,
A 2010 report by Dr. Chan and colleauges37 observed 22 RPE tears in a cohort of 1,002 evaluable eyes (2.2 percent) with neovascular AMD treated with bevacizumab, all of which occurred in the subgroup of 123 eyes with a pre-existing PED (17.9 percent). There was no statistically significant difference in the pre- and post-treatment vision in these 22 eyes despite the fact that only six (27.3 percent) continued to received anti-VEGF therapy. Recently, Dr. Cunning-ham and colleagues79 examined the incidence of RPE tears in the ANCHOR,
Collectively, these studies suggest that the overall incidence of RPE tears in eyes with neovascular AMD is similar regardless of whether an anti-VEGF agent is used, and ranges from 2 to 6 percent in overall AMD cohorts vs. 12 to 25 percent in those patients with a pre-existing PED. In addition, it appears that patients receiving anti-VEGF therapy are more likely to develop a tear earlier in the course of therapy than untreated patients, most probably related to the accelerated involution induced by VEGF inhibition.37
Figure 2B repinted with permission from Am J Ophthal 2004; 138:489-492. Figure 4 reprinted with permission from
Ophthalmic Surg Lasers Imaging. 2009;40(6):570-575.
1. Jager RD, Aiello LP,
2. Peyman GA, Lad EM, Moshfeghi DM. Intravitreal injection of therapeutic agents. Retina 2009;29:875-912,
3. Sampat KM, Garg SJ. Complications of intravitreal injections. Curr Opin Ophthalmol 2010;21:178-183.
4. Eifrig CW, Flynn HW Jr., Scott IU, Newton J. Acute-onset postoperative endophthalmitis: Review of incidence and visual outcomes (1995-2001). Ophthalmic Surg Lasers 2002;33:373-378.
5. Chen SD, Lochhead J, McDonald B, Patel CK. Pseudohypopyon after intravitreal triamcinolone injection for the treatment of pseudophakic cystoids macular oedema. Br J Ophthalmol 2004;88:843-844.
6. Moshfeghi AA, Scott IU, Flynn HW Jr, Puliafito CA. Pseudohypopyon after intravitreal triamcinolone acetonide injection for cystoids macular edema. Am J Ophthalmol 2004;138:489-492.
7. Simon S, Gray T, Dhanapala M, Gilhotra J. Pseudohypopyon following intravitreal triamcinolone acetonide injection in a phakic eye. Clin Exp Ophthalmol 2010;38:76-77.
8. Wickremasinghe SS, Michalova K, Gilhotra J, Guymer RH, et al. Acute intraocular inflammation after intravitreous injections of bevacizumab for treatment of neovascular age-related macular degeneration. Ophthalmology 2008;115:1911-5.
9. Mezad-Koursh D, Goldstein M, Heilwail G, Zayit-Soudry S, et al. Clinical characteristics of endophthalmitis after an injection of intravitreal antivascular endothelial growth factor. Retina 2010;30:1051-1057.
10. Gragoudas ES, Adamis AP, Cunningham ET Jr., Feinsod M, Guyer DR;VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351:2805-16.
11. VEGF Inhibition Study in Ocular Neovascularization (VISION) Clinical Trial Group, D'Amico DJ, Masonson HN, Patel M, Adamis AP, Cunningham ET Jr., et al. Pegaptanib sodium for neovascular age-related macular degeneration: two-year safety results of the two prospective, multicenter, controlled clinical trials. Ophthalmology 2006;113:992-1001.
12. Singerman LJ, Masonson H, Patel M, Adamis AP, et al. Pegaptanib sodium for neovascular age-related macular degeneration: Third-year safety results of the VEGF Inhibition Study in Ocular Neovascularisation (VISION) trial. Br J Ophthalmol 2008;92:1606-1611.
13. Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, et al;MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419-1431.
14. Regillo CD, Brown DM, Abraham P, Yue H, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol 2008;145:239-248.
15. Antoszyk AN, Tuomi L, Chung CY, Singh A;FOCUS Study Group. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): Year 2 results. Am J Ophthalmol 2008;145:862-874.
16. Brown DM, Michels M,
17. Boyer DS, Heier JS, Brown DM, Francom SF, et al. A phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology 2009;116:1731-1739.
18. Fung AE, Rosenfeld PJ, Reichel E. The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophthalmol 2006;90:1344-1349.
19. Jonas JB, Spandau UH, Rensch F, von Baltz S, et al. Infectious and noninfectious endophthalmitis after intravitreal bevacizumab. J Ocul Phmar Ther 2007;23:240-242.
20. Mason JO III, White MF, Feist RM, Thomley ML, et al. Incidence of acute onset endophthalmitis following intravitreal bevacizumab (Avastin) injection. Retina 2008;28:564-567.
21. Angulo Bocco MC, Glacet-Bernard A, Zourdani A, Coscas G, Soubrane G. Intravitreous injection: retrospective study on 2028 injections and their side effects. J Fr Ophthalmol 2008;31:693-698.
22. Wu L, Martínez-Castellanos MA, Quiroz-Mercado H, Arevalo JF, et al. Pan American Collaborative Retina Group (PACORES). Twelve-month safety of intravitreal injections of bevacizumab (Avastin): Results of the Pan-American Collaborative Retina Study Group (PACORES). Graefe's Arch Clin Exp Ophthalmol 2008;246:81-87.
23. Moshfeghi AA, Flynn HW Jr., Murray TG, Rosenfeld PJ, et al. Endophthalmitis following intravitreal injections is very rare in the anti-VEGF era. Invest Ophthalmol Vis Sci 2008;49: E-Abstract 2867.
24. Fintak DR, Shah GK, Blinder KJ, Regillo CD, et al. Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina 2008;28:1395-1399.
25. Wong LJ, Desai RU, Jain A, Feliciano D, et al. Surveillance for potential adverse events associated with the use of intravitreal bevacizumab for retinal and choroidal vascular disease. Retina 2008;28:1151-1158.
26. El-Ashry MF, Dhillon B. The article by Fintak et al on the incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina 2009;29:720-721.
27. Pilli S, Kotsolis A, Spaide RF, Slakter J, et al. Endophthalmitis associated with intravitreal anti-vascular endothelial growth factor therapy injections in an office setting. Am J Ophthalmol 2008;145:879-882.
28. Diago T, McCannel CA, Bakri SJ, Pulido JS, et al. Infectious endophthalmitis after intravitreal injection of antiangiogenic agents. Retina 2009;29:601-605.
29. Klein KS, Walsh MK, Hassan GS, Halperin LS, et al. Endophthalmitis after anti-VEGF injections. Ophthalmology 2009;116:1225.e1.
30. Ladas ID, Karagiannis DA, Rouvas AA, Kotsolis AI, et al. Safety of repeat intravitreal injections of bevacizumab versus ranibizumab: Our experience after 2,000 injections. Retina 2009;29:313-318.
31. Lima LH, Zweifel SA, Engelbert M, Sorenson JA, et al. Evaluation of safety for bilateral same-day intravitreal injections of antivascular endothelial growth factor therapy. Retina 2009;29:1213-1217.
32. Bhavsar AR, Googe JM Jr., Stockdale CR, Bressler NM, et al;Diabetic Retinopathy Clinical Research Network. Risk of endophthalmitis after intravitreal drug injection when topical antibiotics are not required: The diabetic retinopathy clinical research network laser-ranibizumab-triamcinolone clinical trials. Arch Ophthalmol 2009;127:1581-1583.
33. Aiello LP, Brucker AJ, Chang S, Cunningham ET Jr., et al. Evolving guidelines for intravitreous injections. Retina 2004:S3-S19.
34. Moshfeghi AA, Flynn HW Jr. Complications of IVTA injection. Review of Ophthalmology 2004;December, 64-67.
35. Scott IU, Flynn HW Jr. Reducing the risk of endophthalmitis following intravitreal injections. Retina 2007;27:10-12.
36. Chang LK, Sarraf D. Tears of the retinal pigment epithelium: An old problem in a new era. Retina 2007;27:523-534.
37. Chan CK, Abraham P, Meyer CH, Kokame GT, et al. Optical coherence tomography-measured pigment epithelial detachment height as a predictor for retinal pigment epithelial tears associated with intravitreal bevacizumab injections. Retina 2010;30:203-211.
38. Hoskin A, Bird AC, Sehmi K. Tears of detached retinal pigment epithelium. Br J Ophthalmol 1981;65:417-422.
39. Gass JD. Pathogenesis of tears of the retinal pigment epithelium. Br J Ophthalmol 1984;68:513-519.
40. Gass JDM. Serous retinal pigment epithelium detachment with a notch: A sign of occult choroidal neovascularization. Retina 1984;4:205-220.
41. Cantrill HL, Ramsay RC, Knobloch WH. Rips in the pigment epithelium. Arch Ophthalmol 1983;101:1074-1079.
42. Casswell AG, Kohen D, Bird AC. Retinal pigment epithelial detachments in the elderly: classification and outcome. Br J Ophthalmol 1985;69:397–403.
43. Goldstein BG, Pavan PR. 'Blow-outs' in the retinal pigment epithelium. Br J Ophthalmol 1987;71:676-681.
44. Chuang EL, Bird AC. Repair after tears of the retinal pigment epithelium. Eye 1988;2:106-113.
45. Coscas G, Koenig F, Soubrane G. The pretear characteristics of pigment epithelial detachments. A study of 40 eyes. Arch Ophthalmol 1990;108:1687-1693.
46. Leitritz M, Gelisken F, Inhoffen W, Voelker M, Ziemssen F. Can the risk of retinal pigment tears after bevacizumab treatment be predicted? An optical coherence tomography study. Eye 2008;22:1504-1507.
47. Chiang A, Chang LK, Yu F, Sarraf D. Predictors of anti-VEGF-associated retinal pigment epithelial tear using FA and OCT analysis. Retina 2008;28:1265-1269.
48. Yeo JD, Marcus S, Murphy RP. Retinal pigment epithelial tears. Patterns and prognosis. Ophthalmology 1988;95:8-13.
49. Gass JDM. Retinal pigment epithelial rip during krypton red laser photocoagulation. Am J Ophthalmol 1984;98:700-706.
50. Gelisken F, Inhoffen W, Partsch M, Schneider U, Kreissig I. Retinal pigment epithelial tear after photodynamic therapy for choroidal neovascularization. Am J Ophthalmol 2001;131:518-520.
51. Pece A, Introini U, Bottoni F, Brancato R. Acute retinal pigment epithelial tear after photodynamic therapy. Retina 2001;21:661-665.
52. Srivastava SK, Sternberg P Jr. Retinal pigment epithelial tear weeks following photodynamic therapy with verteporfin for choroidal neovascularization secondary to pathologic myopia. Retina 2002;22:669-771.
53. Arnold JJ, Blinder KJ, Bressler NM, Bressler SB, Burdan A, Haynes L, Lim JI, Miller JW, Potter MJ, Reaves A, Rosenfeld PJ, Sickenberg M, Slakter JS, Soubrane G, Strong HA, Stur M;Treatment of Age-Related Macular Degeneration with Photodynamic Therapy Study Group;Verteporfin in Photodynamic Therapy Study Group. Acute severe visual acuity decrease after photodynamic therapy with verteporfin: Case reports from randomized clinical trials-TAP and VIP report no. 3. Am J Ophthalmol 2004;137: 683-96.
54. Goldstein M, Heilweil G, Barak A, Loewenstein A. Retinal pigment epithelial tear following photodynamic therapy for choroidal neovascularization secondary to AMD. Eye 2005;19:1315-132.
55. Michels S, Aue A, Simader C, Geitzenauer W, et al. Retinal pigment epithelium tears following verteporfin therapy combined with intravitreal triamcinolone. Am J Ophthalmol 2006;141:396-398.
56. Sutter FK, Kurz-Levin MM, Scherrer M, Barthelmes D, et al. Intravitreal triamcinolone acetonide for serous retinal pigment epithelial detachments in exudative age-related macular degeneration. Klin Monatsbl Augenheilkd 2007;224:297-299.
57. Dhalla MD, Blinder KJ, Tewari A, Hariprasad SM, Apte RS. Retinal epithelial pigment tear following intravitreal pegaptanib sodium. Am J Ophthalmol 2006;141:751-753.
58. Singh RP, Sears JE. Retinal pigment epithelial tears after pegaptanib injection for exudative age-related macular degeneration. Am J Ophthalmol 2006;142:160-162.
59. Chang LK, Flaxel CJ,
60. Carvounis PE, Kopel AC, Benz MS. Retinal pigment epithelium tears following ranibizumab for exudative age-related macular degeneration. Am J Ophthalmol 2007;143:504-505.
61. Bakri SJ,
62. Chan CK, Lin SG. Retinal pigment epithelial tear after ranibizumab therapy for subfoveal fibrovascular pigment epithelial detachment. Eur J Ophthalmol 2007;17:674-676.
63. Smith RB, Kraus CL, Apte RS. Retinal pigment epithelial tears in ranibizumab-treated eyes. Retina 2009;29:335-339.
64. Konstantinidis L, Ambresin A, Zografos L, Mantel I. Retinal pigment epithelium tears after intravitreal injection of ranibizumab for predominantly classic neovascular membranes secondary to age-related macular degeneration. Acta Ophthalmol 2009;Jul 14. Epub ahead of print.
65. Kiss C, Michels S, Prager F, Geitzenauer W, Schmidt-Erfurth U. Retinal pigment epithelium tears following intravitreal ranibizumab therapy. Acta Ophthalmol Scand 2007;85:902-903.
66. Meyer CH, Mennel S, Schmidt JC, Kroll P. Acute retinal pigment epithelial tear following intravitreal bevacizumab (Avastin) injection for occult choroidal neovascularization secondary to age related macular degeneration. Br J Ophthalmol 2006;90:1207-1208.
67. Shah CP, Hsu J, Garg SJ, Fischer DH, Kaiser R. Retinal pigment epithelial tear after intravitreal bevacizumab injection. Am J Ophthalmol 2006;142:1070-1072.
68.
69. Nicolo M, Ghiglione D, Calabria G. Retinal pigment epithelial tear following intravitreal injection of bevacizumab (Avastin). Eur J Ophthalmol 2006;17:770-773.
70. Garg S, Brod R, Kim D, Lane RG, Maguire J, Fischer D. Retinal pigment epithelial tears after intravitreal bevacizumab injection for exudative age-related macular degeneration. Clin Experiment Ophthalmol 2008;36:252-256.
71. Arias L, Caminal JM, Rubio M, Pujol O, Arruga J. Retinal pigment epithelial tears after intravitreal bevacizumab injection for predominantly classic choroidal neovascularization. Eur J Ophthalmol 2007;17:992-995.
72. Shaikh S, Olson JC, Richmond PP. Retinal pigment epithelial tears after intravitreal bevacizumab injection for exudative age-related macular degeneration. Indian J Ophthalmol 2007;55:470-472.
73. Cleary CA, Jungkim S, Ravikumar K, Kelliher C, et al. Intravitreal bevacizumab in the treatment of neovascular age-related macular degeneration, 6- and 9-month results. Eye 2008;22:82-86.
74. Kook D, Wolf A,
75. Ronan SM, Yoganathan P, Chien FY,
76. Weinberger AWA, Thiel M, Mohammadi B, Theofylaktopoulos I, et al. Retinal pigment epithelium tears after intravitreal bevacizumab in pigment epithelium detachment. Am J Ophthalmol 2007;44:294-296.
77. Chan CK, Meyer CH, Gross JG, Abraham P, et al. Retinal pigment epithelial tears after intravitreal bevacizumab injection for neovascular age-related macular degeneration. Retina 2007;27:541-551.
78. Moroz I, Moisseiev J, Alhalel A. Optical coherence tomography predictors of retinal pigment epithelial tear following intravitreal bevacizumab injection. Ophthalmic Surg Lasers Imaging. 2009;40(6):570-575.
79. Cunningham ET Jr., Feiner L, Chung CY, Rundle AC, Tuomi L. Incidence of retinal pigment epithelial (RPE) tears after intravitreal ranibizumab injection for neovascular age-related macular degeneration. Retina Congress 2009
80. Chan CK, Lin SG, Retinal pigment epithelial tear after ranibizumab therapy for subfoveal fibrovascular pigment epithelial detachment. Eur J Ophthalmol 2007;17:674-676.
81. Gale J, Cheung J. Resolution of subretinal fluid associated with a spontaneous retinal pigment epithelial tear after intravitreal ranibizumab injection. Can J Ophthalmol 2009;44:345-346.
82. Lesniak SP, Fine HF, Prenner JL, Roth DB. Long-term follow-up of spontaneous retinal pigment tears in age-related macular degeneration treated with anti-VEGF therapy. Eur J Ophthalmol 2010;20: Jul 7. [Epub ahead of print].
83. Sarraf D, Reddy S, Chiang A, Yu F. A new grading system for retinal pigment epithelial tears. Retina 2010;30:1039-1045.



