Allen Chiang, MD
Julia A. Haller, MD
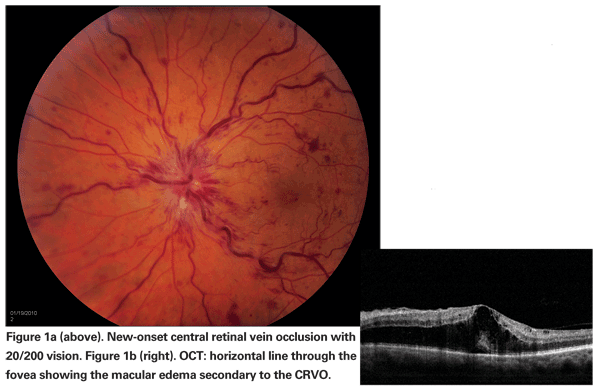
Visual morbidity in branch or central retinal vein occlusion stems from the development of macular ischemia, retinal or optic disc neovascularization, neovascular glaucoma and/or persistent macular edema. Of these, macular edema is a frequent and major cause of visual loss (See Figure 1).1,2
While grid laser photocoagulation was shown to be of benefit for treating persistent macular edema due to BRVO in the Branch Vein Occlusion Study (BVOS), no similar advantage could be documented for this therapy in the Central Vein Occlusion Study (CVOS).3,4 Various alternative treatment modalities for CRVO have been studied, but none has achieved proven efficacy.5,6
Intravitreal pharmacotherapy, including use of vascular endothelial growth factor inhibitors and corticosteroids, has increasingly become a staple of clinical practice with accumulating data to inform this approach. Clinical trials of intravitreal ranibizumab for BRVO (BRAVO) and CRVO (CRUISE) have reported six-month results showing substantive visual benefits to monthly injections. With regard to corticosteroids, the results of a Phase III trial of intravitreal triamcinolone acetonide were recently published and a sustained-release intravitreal dexamethasone implant (Ozurdex) was approved by the Food and Drug Administration for the treatment of macular edema due to either BRVO or CRVO.
Intravitreal Triamcinolone Acetonide Therapy
Vascular permeability resulting in macular edema is due to a breakdown of the blood-retina barrier, a process with multifactorial mediators including a significant role for VEGF.7 Dampening the effects of VEGF has been shown to decrease the formation of macular edema, and since inflammation also plays a role in the pathophysiology of vein occlusion, corticosteroids may confer an added advantage.8 Furthermore, they act to stabilize vascular endothelial cell tight junctions and down-regulate VEGF expression.9,10
Recently, a multicenter, randomized Phase III trial comparing the safety and efficacy of IVTA to treat vision loss associated with macular edema secondary to CRVO (SCORE-CRVO) identified the superior efficacy of two doses of IVTA (1 mg and 4 mg) compared with observation.11 Approximately 26 percent of participants in each IVTA group achieved a gain in visual acuity of ¡Ý15 ETDRS letters from baseline to month 12, compared with 7 percent of those observed. The odds of achieving this were five times greater with IVTA, with no difference between the 1-mg and 4-mg groups, although complications including elevated IOP and cataract were more common in the 4-mg group.
Mean visual acuity was better in the triamcinolone groups than the observation group for all time points through 12 months. Interestingly, visual improvement was only moderately correlated with OCT-measured retinal thickness; in fact at 12 months no difference existed between the groups. Investigators postulated this discordance could be due to a neuroprotective or anti-inflammatory effect of the corticosteroids.
From 12 months to two years, cataract formation may have attenuated the effect of IVTA on the mean change in visual acuity, yet the results still favored the two triamcinolone groups. Notably, the frequency of cataract and glaucoma surgery was similar between the 1-mg IVTA and observation groups, conferring the 1-mg group a superior safety profile over the 4-mg group. Thirty-five percent of the 4-mg group required IOP-lowering medication compared to only 20 percent of the 1-mg group. There were no cases of retinal detachment or either infectious or noninfectious endophthalmitis. Based on these results, treatment with 1 mg IVTA should be considered for patients with vision loss due to CRVO-related macular edema following the four-month retreatment interval used in this study for one year, possibly two, unless contraindicated.
In contrast, a randomized clinical trial (SCORE-BRVO) comparing the efficacy and safety of IVTA for BRVO-associated macular edema with standard care (grid laser) showed no difference in vision between laser and the triamcinolone groups (1 mg or 4 mg) at 12 months.12 Therefore the collaborators concluded that laser remains the benchmark of care. As in SCORE-CRVO, the highest rate of adverse events occurred in the 4-mg IVTA group.
Of course the recent studies of steroid and anti-VEGF therapy for macular edema due to BRVO did not randomize eyes to ranibizumab versus IVTA, so it is difficult to compare them. Numerous differences exist between studies, including the baseline characteristics of eyes enrolled. For example, the mean duration of macular edema prior to enrollment in SCORE-BRVO was four months, with only 37 percent of eyes with less than three months duration. This is commensurate with the fact that BRVO-related macular edema is often observed initially either due to hemorrhage precluding laser or the potential of spontaneous improvement. Yet in BRAVO, subjects were enrolled earlier, with 51.5 percent of the 0.3-mg ranibizumab group and 57 percent of the 0.5-mg group less than three months out from diagnosis of BRVO. Whether this relative delay in treatment affected the ultimate outcome is uncertain, but increased interest in earlier intervention has clearly developed, fueled by data from these studies and the Ozurdex trials.
Sustained-Release Dexamethasone
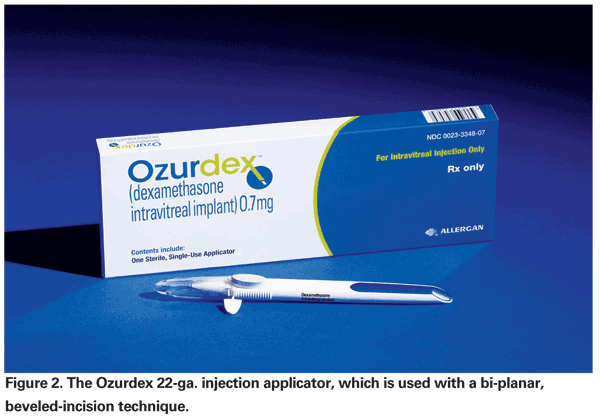
Via mechanisms similar to triamcinolone, dexamethasone is capable of reducing vascular permeability and leukocyte accumulation through multiple pathways, including VEGF and ICAM-1 expression.13 While dexamethasone is a more potent corticosteroid, its half-life of approximately three hours renders intravitreal injection an impractical delivery method. A proprietary posterior segment drug delivery system (DDS) consisting of a biodegradable copolymer of lactic and glycolic acids was developed to circumvent this problem. Gradual polymer degradation into water and CO2 allows for sustained release of dexamethasone. In preclinical studies it has been detected up to six months following implantation (Allergan data on file, 2006-2007). This DDS can now be administered in the office using a novel 22-ga. applicator recently evaluated by Dr. Haller and colleagues (See Figure 2).14
In 2007 Baruch Kuppermann, MD, and co-investigators reported the efficacy of an earlier iteration of this DDS with regard to improved visual acuity, macular thickness and fluorescein leakage in a Phase II study of patients with persistent macular edema (>90 days) from various causes including vein occlusion.15 More recent is the release of Phase III data and consequent FDA-approval of a sustained-release 700-mcg dexamethasone implant (Ozurdex) for the specific indication of macular edema following retinal vein occlusion.
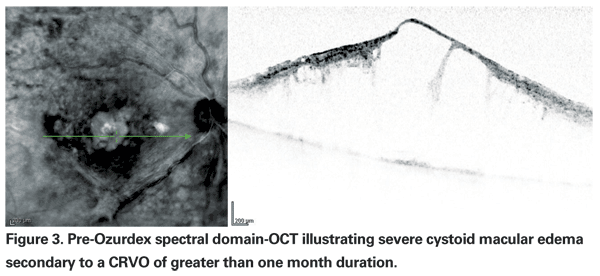
Ozurdex was studied in two randomized, multicenter, masked, sham-controlled, parallel-group Phase III trials with about 1,300 patients with macular edema due to CRVO (one-third) or BRVO (two-thirds) and followed for one year, with a primary endpoint at six months. Randomization was to one of three groups: 700-mcg implant, 350-mcg implant or sham. Data presented by Dr. Haller at the 2009 AAO Subspecialty Retina Day demonstrated that with Ozurdex the time to gain of a three-line improvement in BCVA was significantly faster compared to sham, with attainment of this goal within the first two months in approximately 20 to 30 percent of patients (compared to 7 to 12 percent of sham-treated patients) and persistence through day 180. (Haller JA. Six-month randomized controlled clinical trial of an intravitreal dexamethasone implant in macular edema associated with retinal vein occlusion. AAO Subspecialty Retina Day 2009.)
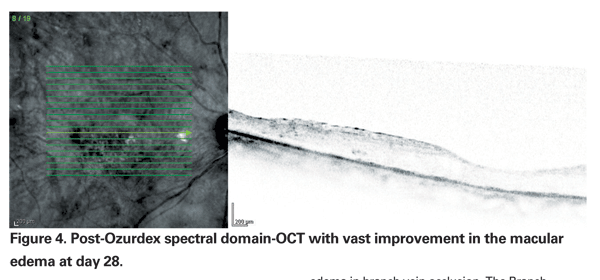
Specifically, improvement peaked at day 60, with 29.3 percent of patients in each Ozurdex group (350 mcg and 700 mcg) gaining three or more lines compared to the 11.3 percent of patients in the sham group (p<0.001) (See Figures 3 & 4). At 180 days, a cumulative 41 percent of treated patients had gained >three lines of vision, compared with 23 percent of sham patients. Interestingly, signals that the duration of disease may be important included data from a post hoc analysis of a subset of participants with macular edema for less than 90 days showing that they achieved even better visual outcomes with Ozurdex (40 to 50 percent gained >three lines at day 60). However, the majority (>80 percent) of eyes in the study had macular edema for >three months; roughly a third for >six months.
With regard to safety, 0.2 percent of patients in either dexamethasone group had intraocular pressure of >35 mmHg and 1.2 percent had IOP >25 mmHg at six months. In those who received Ozurdex, 29.7 percent required IOP-lowering medication at day 90, but in all groups IOP returned to baseline by day 180. Only five eyes (0.7 percent) required surgery for IOP control (three for neovascular glaucoma). Rates of cataract progression out to one year did not differ significantly between the groups.
Different Outcomes, Time Points
In the Ozurdex trial the primary outcome measure was the time to achieve a ¡Ý15-letter improvement in visual acuity. In SCORE-CRVO it was the proportion of those with ¡Ý15-letter gain in visual acuity at 12 months. In contrast, CRUISE looked at the mean visual acuity at the month six visit following monthly intravitreal ranibizumab injections.
In spite of disparities amongst studies in terms of baseline characteristics, protocol design and endpoints, a limited comparison for CRVO-associated macular edema is possible by analyzing the absolute difference and the number needed to treat based on the available data. In SCORE-CRVO, regarding the primary outcome measure, there was an absolute difference of +20 percent for 1-mg IVTA over observation, or a number needed to treat (NNT) of five patients. In CRUISE, the absolute difference of either 0.3-mg or 0.5-mg ranibizumab over observation was approximately +30 percent, an NNT of 3.3. For either dose of Ozurdex, the absolute difference at month six was +18 percent over observation, an NNT of 5.5.
With this approach, these modalities appear somewhat comparable, and might even be more so if shorter-duration occlusions were used for the steroid calculations, comparable to baseline characteristics in CRUISE. Less can be concluded for BRVO-associated macular edema since there was no comparison of ranibizumab to laser in BRAVO and no apparent difference between IVTA and laser in SCORE-BRVO.
Macular edema remains a significant cause of disabling vision loss following retinal vein occlusion. The SCORE-CRVO study supports 1-mg IVTA for CRVO-associated macular edema. While SCORE-BRVO did not demonstrate a significant difference between IVTA and standard grid laser, both would be superior to the natural history of the disease if historical controls from the untreated arm of the BVOS are used (no eyes were randomized to observation in SCORE-BRVO).
Ozurdex is the first FDA-approved treatment for macular edema secondary to BRVO or CRVO. In clinical trials over a 12-month period, a rapid onset and persistent effect was demonstrated, with very few discontinuations due to adverse events. Post hoc data analysis showed that earlier treatment may be associated with greater visual improvement across subgroups, and suggests that further investigation into the impact of early therapy may be worthwhile. As well, the intriguing suggestion that factors other than thinning of the retina may underlie the visual improvement associated with the use of steroids deserves further exploration.
In addition to corticosteroid therapy, anti-VEGF agents are a treatment consideration. CRUISE and BRAVO lend support to the utility of ranibizumab, the short-term drawback being, of course, the frequent injections. As well, no formal comparison to laser was made in BRAVO, so its relative utility is unknown. VEGF Trap (Regeneron), a soluble VEGF receptor fusion protein that binds all forms of VEGF-A and placental growth factor, is currently under investigation for CRVO in the COPERNICUS and GALILEO trials and may add to an expanding treatment armamentarium. Other potential avenues of study include multimodality therapy, perhaps with combination regimens and laser photocoagulation.
Decades after the release of the BVOS and CVOS results, new therapies offer promise to patients and clinicians struggling with the treatment of retinal venous occlusive disease. Although many pieces of the therapeutic puzzle remain to be sorted out, there is renewed investigative interest in these disorders, spurred by emerging data from a series of well-designed trials recently published or in press.
Dr. Chiang is a vitreoretinal fellow at the Wills Eye Institute. Dr. Haller is Ophthalmologist-in-Chief of the Wills Eye Institute and professor and chair of the department of ophthalmology at
1. Snelling JB, Nisbet RM. Retinal branch vein occlusion. Ann Ophthalmol 1981;13:1273-6.
2. Baseline and early natural history report. The Central Vein Occlusion Study. Arch Ophthalmol 1993;111:1087-95.
3. Argon laser photocoagulation for macular edema in branch vein occlusion. The Branch Vein Occlusion Study Group. Am J Ophthalmol 1984;98:271-82.
4. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The Central Vein Occlusion Study Group M report. Ophthalmology 1995;102:1425-33.
5. Hayreh SS. Management of central retinal vein occlusion. Ophthalmologica 2003;217:167-88.
6. Mohamed Q, McIntosh RL, Saw SM, et al. Interventions for central retinal vein occlusion: An evidence-based systematic review. Ophthalmology 2007;114:507-19, 524.
7. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994;331(22):1480-7.
8. Lee HB, Pulido JS,
9. Tong JP, Lam DS, Chan WM, et al. Effects of triamcinolone on the expression of VEGF and PEDF in human retinal pigment epithelial and human umbilical vein endothelial cells. Mol Vis 2006;12:1490-5.
10. Felinski EA, Antonetti DA. Glucocorticoid regulation of endothelial cell tight junction gene expression: Novel treatments for diabetic retinopathy. Curr Eye Res 2005; 30(11):949-57.
11. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol 2009;127:1101-14.
12. Scott IU, Ip MS, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: The Standard Care vs. Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol 2009;127:1115-28.
13. Wang K, Wang Y, Gao L, et al. Dexamethasone inhibits leukocyte accumulation and vascular permeability in retina of streptozotocin-induced diabetic rats via reducing vascular endothelial growth factor and intercellular adhesion molecule-1 expression. Biol Pharm Bull 2008;31(8):1541-6.
14. Haller JA, Dugel P, Weinberg DV, et al. Evaluation of the safety and performance of an applicator for a novel intravitreal dexamethasone drug delivery system for the treatment of macular edema. Retina 2009;29:46-51.
15. Kuppermann BD, Blumenkranz MS, Haller JA, et al. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol 2007;125:309-17.



