This summer, the U.S. Food and Drug Administration has approved two new minimally invasive glaucoma surgery devices designed to help lower intraocular pressure by improving aqueous outflow through the trabecular meshwork: the iStent inject (Glaukos), approved in June, and the Hydrus (Ivantis), approved in August. Here, two surgeons familiar with both devices share their experience and advice regarding these new options for addressing mild to moderate glaucoma.
Optimizing the TM Pathway
“To date there have been five prospective, randomized trials comparing cataract surgery alone to cataract surgery performed along with one of the procedures that are considered to be MIGS,” notes Thomas W. Samuelson, MD, a founding partner at Minnesota Eye Consultants in Minneapolis and an adjunct professor of ophthalmology at the University of Minnesota. (Dr. Samuelson was an investigator in the iStent inject trial.) “They include the original iStent trial and the subsequent iStent inject trial; the CyPass COMPASS trial; and two prospective, randomized trials using the Hydrus—one in Europe and one in the U.S. One of the important takeaways from all of those trials has been that cataract surgery alone is a meaningful intervention for patients with mild to moderate glaucoma. That’s important, because that’s the foundation on which MIGS rests, in my opinion.
“While the exact mechanism is not clear, we think that removing the cataract is helping physiologic outflow,” he continues. “While speculative, I believe it is mechanical. Removing the lens allows the iris, angle and ciliary zone complex to assume a more posterior position, and that improves outflow. So, we start with that favorable occurrence and we try to add to it without disrupting that improvement in normal physiology.
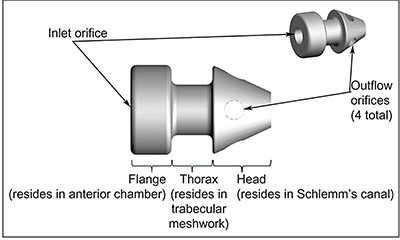 |
| The iStent inject allows the surgeon to inject two stents into Schlemm’s canal instead of one, increasing the likelihood of ending up adjacent to a collector channel. Surgeons also report that it’s easier to use than the original iStent. |
 |
“I think that’s where procedures like iStent and Hydrus and some of the other canal-based procedures really shine; they’re also improving physiologic outflow,” he continues. “My philosophy is that if I can augment the pressure-lowering caused by the cataract surgery, in a fashion that’s synergistic with whatever the cataract has done, that’s an added bonus. With canal-based procedures you’re retaining the outflow pathway that you were born with, the one that’s most physiological. In contrast, combining cataract surgery with a procedure that diverts fluid away from the physiologic pathway—such as a procedure that diverts aqueous across the sclera into a bleb—seems counterintuitive to me, at least for patients with mild to moderate glaucoma undergoing concurrent phacoemulsification.”
What does the data have to say? The recent iStent inject study was conducted at 41 sites with 380 subjects receiving phaco plus iStent inject and 118 receiving phaco alone. Efficacy endpoints at 24 months were a ≤20-percent reduction in diurnal IOP (primary endpoint) and the mean reduction in diurnal IOP (secondary endpoint). At 24 months, 75.3 percent of the iStent inject cohort achieved a 20-percent or greater reduction in unmedicated IOP, compared to 61.9 percent of the phaco-only cohort. At 24 months, the iStent inject cohort had a mean unmedicated IOP reduction of 6.9 mmHg, compared to a 5.4mmHg reduction in the phaco-only cohort. During the 24-month follow-up period, the overall rate of adverse events for the iStent inject group was similar to cataract surgery only.
The Hydrus trial (HORIZON) was a prospective, multicenter randomized trial in which 369 individuals received a Hydrus shunt in addition to phaco, while 187 received phaco only. The primary endpoint at 24 months was a 20-percent reduction in diurnal IOP; the secondary endpoint was the change in mean diurnal IOP. At 24 months, 77.2 percent of the Hydrus group had at least a 20-percent drop in DIOP; 57.8 percent of the phaco-only group achieved that. The mean change in DIOP was -7.6 mmHg at 24 months for the Hydrus group and -5.3 mmHg for the phaco-only group.1
Updating the iStent
Alan Crandall, MD, a clinical professor and senior vice chair of ophthalmology, and director of glaucoma and cataract, in the Department of Ophthalmology at the Moran Eye Center, University of Utah, participated in the analysis of the data from the iStent inject trial, and he’s been using the iStent inject for several months. “Everyone thought the original iStent would be very easy for non-glaucoma surgeons to use,” he notes. “However, the number of people using it who are not glaucoma specialists has dropped, because it’s not as easy as they thought. It’s a little tricky to get into the canal, and you always have the issue of deciding where to put the stent.
“The new iStent model is different, both because it gives you two stents and because it’s easier to put in,” he continues. “The advantage of putting two in is that you’re more likely to get access to at least one outflow channel, and the trial data supports this. In fact, outside the United States everybody uses two or three iStents to get a consistently lower IOP. Although the iStent inject study design was somewhat different from the original iStent trial, the data showed a more consistent and long-lasting result than was achieved with the original device—at least for the two or three years of follow-up that’s been measured so far. The IOP lowering is at least one or two points greater than what we get with a single iStent.”
Dr. Samuelson agrees that the iStent inject trial confirmed the efficacy of the new device. “In the iStent inject trial, the cataract-alone group had favorable IOP reductions; but the cataract plus the iStent inject lowered pressure better and reduced the need for medications better than the control arm,” he says. “Furthermore, it did so in a very safe fashion; there were no significant differences in the safety measures between the groups.”
Dr. Samuelson notes that safety is one of the hallmarks of the iStent approach. “I think once you become facile with these MIGS canal devices, especially the iStent, you can look patients in the eye and tell them that the greatest risk is the risk of disappointment, the possibility that this might not get them all the way to your pressurelowering goal,” he says. “That [level of safety] isn’t true for all MIGS procedures, but I think it’s true for the iStent. Its greatest attribute is how safe it is. Other procedures may prove to be more efficacious, but I’m not aware of any that are safer.”
Dr. Crandall says the company is thinking about creating a next-generation iStent device that will contain three stents. “Having just gotten the iStent inject through regulatory, I’m sure they’ll stick with the iStent inject for now,” he notes. “Still, the idea of adding more stents is an obvious option to consider. It’s possible that it would produce even greater pressurelowering.”
Location, Location, Location
“One of the nice things about both of the new canal devices we’re expecting in 2018—the iStent inject and the Hydrus—is that they take some of the guesswork out of placing the stents,” Dr. Samuelson explains. “Using the original iStent meant placing a single stent, so the surgeon had to choose the most favorable place to put it, a process that has become known as ‘intelligent placement.’ Many surgeons have found this challenging, which is completely understandable. In contrast, the iStent inject employs a twostent strategy. If you put both stents in the inferonasal quadrant, where the number of collector channels is greatest, you’re very likely to end up in close proximity to a collector channel. Likewise with Hydrus. It’s 8 mm long, so you can put it into the inferonasal quadrant and cover the whole quadrant.
“In essence, the issue of where to put the stent in the angle goes away to some extent if you’re using the iStent inject or the Hydrus,” he says. “That’s a nice advantage over the original iStent concept. So if you’ve been sitting on the sidelines because you found the original iStent procedure a bit tricky or couldn’t figure out the best location for the stent, you should find the new options easier to use.”
Dr. Samuelson notes that the insertion of the stent into the canal is also easier with the iStent inject. “My learning curve for the original iStent occurred as an investigator in the FDA trial,” he says. “Doing my first 20 cases or so, I didn’t have nearly as much confidence that I was getting the stent into the canal every time [as I did later]. In contrast, the new version is easier to implant; it uses a different insertion technique. Instead of circumferential placement requiring a lateral motion and checking the depth of the stent, this is a straight-in insertion. I suspect that surgeons will like the new design and find it easier to use than the original iStent.”
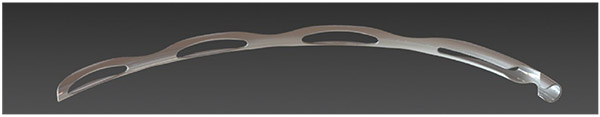 |
The Hydrus Microstent features a tri-modal mechanism: The tail end, sitting in the anterior chamber, provides a direct inlet into Schlemm’s canal; the body of the stent maintains the patency of the canal; and the device tensions the canal tissue, improving outflow through it. |
 |
Dr. Crandall, however, notes one drawback to the new way of implanting the stent used in the iStent inject. “Although the new device is easier to use, the fact that you’re injecting the stent rather than inserting it means that it can easily go into the wrong spot,” he points out.
“If the original stent was not in the canal, it was pretty obvious; then you could make another attempt to get it in. But the new device can be inadvertently injected just above or below the canal, and you won’t necessarily realize that it’s not in the canal. If it’s a lightly pigmented eye you could end up too high, close to Schwalbe’s line. In that situation you might suspect that something’s wrong, because you should see blood coming out if you get the stent into the canal. But even in the canal you won’t always see blood, so you might conclude that everything is OK and proceed to place the second stent. If you hit too low you could end up in the ciliary body or right below the canal, and the stent would still go right in.
“The bottom line is that there is still a skill set involved,” he concludes. “To their credit, the Glaukos people know that and they emphasize the need for lots of practice, to make sure surgeons can identify the canal, which is sometimes not easy to do.”
One obvious question is how much better the outcomes might be with two stents implanted rather than one. Dr. Samuelson explains that this question is harder to answer than one might expect. “The reason that’s hard to determine is that since the original iStent trial, the FDA has changed its study design recommendations,” he says. “Now the study design and study power is based on generating two-year data and a three-to-one randomization. In addition, unlike the original iStent trial, more recent MIGS trials include a terminal washout of glaucoma medications to eliminate the confounding effect of those medications on IOP. Those changes make it a little hard to compare the original iStent study to the subsequent MIGS studies, head-to-head.
“Nevertheless, I suspect the improvement in efficacy compared to the original design is not going to be overwhelming,” he says. “The big improvement will be in terms of ease of implantation. I don’t mean to imply that two stents wouldn’t be better than one; I think the data have demonstrated that they are.2 It’s just a little bit difficult to prove that by comparing the results of the original iStent trial to the results of the iStent inject trial, because of the difference in study design.”
Comparing the Options
Since the premise of the iStent inject is achieving improved outflow via better access to the collector channels, surgeons may wonder about the relative merits of the iStent inject compared to the Hydrus, which should provide even greater access to the channels, at least in theory. “The Hydrus has a lot of promise,” says Dr. Samuelson. “The data from the HORIZON trial, which is the pivotal U.S. trial for Hydrus, was extremely favorable—probably the best MIGS data that we’ve seen to date, in terms of the important combination of sustained efficacy and safety. There’s also a randomized, multicenter, international study soon to be published, the COMPARE trial [sponsored by Ivantis], in which Hydrus compared favorably to two iStents.
The advantage of Hydrus is the tri-modal mechanism. First, it provides a direct inlet into the canal because the tail end of the Hydrus resides in the anterior chamber. Second, the main body of the Hydrus, the remaining 7 mm or so, sits within the canal, maintaining its patency. Third, it also tensions the canal tissue, improving physiological outflow.”
Dr. Crandall notes that a recent trial that compared the Hydrus to the iStent used the original iStent, not the iStent inject. “The paper with the data hasn’t been published yet,” he says. “If the Hydrus did better than the single iStent [in that study], that shouldn’t be a surprise; the Hydrus accesses at least 90 degrees of the canal, so its odds of accessing one or more outflow channels is much greater. But there’s no study comparing the Hydrus to the iStent inject.”
“Despite the promise of Hydrus, There will no doubt be proponents of both canal devices,” says Dr. Samuelson. “Some surgeons might prefer the iStent inject because it’s a ‘stealth’ device and the least-tissue-disruptive canal intervention. On the other hand, many surgeons will like the Hydrus design, and the fact that you can directly verify that it’s exactly where you want it to be in the canal because you can see it through the translucent inner wall.
“The only head-to-head data available favors Hydrus (the COMPARE trial), yet some will argue that with the Hydrus you’re manipulating 8 mm of the canal in a very important region,” he continues. “As a result, there could be some fibrosis over time that might not occur with a very stealthy implantation like the iStent inject, which is very tissue-friendly and maintains the normal architecture of the canal as much as possible. The data clearly show that cataract surgery alone improves physiologic function, so one might argue that it makes sense to disrupt the tissue as little as possible while augmenting outflow. For some, that concept might favor an iStent approach. You put in two minimally tissue-disruptive, extremely focal stents, leaving 98 percent of the canal normal, but improved from the phaco effect. Time will tell, but I’m confident that there’s room for more than one canal device, just as we have multiple IOL platforms and phaco machines. “Ultimately, the device that surgeons favor may depend on what long-term data shows in a large population of patients,” he adds. “More comparative data will be forthcoming, and both devices should really improve the MIGS portfolio.”
The Road Ahead
Dr. Crandall says he expects the iStent inject to overshadow the original iStent in the marketplace very quickly. “I don’t think there’s any question about that,” he says. “The inject gives you two shots at hitting an outflow channel, and although it still requires gonioscopy skill and a good understanding of the anatomy to get it into the correct spot, it’s easier to use. In my experience, the Hydrus is a bit easier to insert than the original iStent, but I find the iStent inject to be even easier than the Hydrus. I think the iStent inject will replace the original iStent very quickly.”
Regarding whether these devices might someday be used separately from cataract surgery (i.e., off-label), Dr. Samuelson says he can imagine that happening. “I think the iStent may be a little more dependent on the phaco component of the procedure than another MIGS device such as the CyPass or Hydrus,” he says. “Nevertheless, I see them all as having a role in standalone surgery.”
What about the possibility of a device containing three or more iStent implants? “I would think that implanting more stents might allow you to tap into more collector channels, and in general, the more the better,” he says. “However, there could be a limit at some point to how many are useful, especially in that segment of the population that has more distal disease.”
Dr. Crandall says he believes the new iStent device will eventually be used in the United States independently of cataract surgery as well as in concert with it. “Everywhere else in the world surgeons use the iStent by itself, as they do with most other MIGS devices,” he points out. “In the United States, to get through the regulatory pathway, use of the device had to be combined with cataract surgery to ensure that the outcomes would be impressive enough to have a good chance of approval by the FDA. That was true for Hydrus, too. The iStent will remain in that regulatory category for a while, I expect, but eventually surgeons in the U.S. will start using it by itself. That will also move us in the direction of trials comparing the different MIGS devices head-to-head, which will give us a lot of useful data.” REVIEW
Drs. Crandall and Samuelson both consult for Ivantis and Glaukos.
1. Samuelson TW, Chang DF, Marquis R, et al. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: The HORIZON study. Ophthalmology 2018 Jun 23. pii: S0161-6420(17)33810-1. doi: 10.1016/j.ophtha.2018.05.012. [Epub ahead of print]
2. Belovay GW, Naqi A, Chan BJ, Rateb M, Ahmed II. Using multiple trabecular micro-bypass stents in cataract patients to treat openangle glaucoma. J Cataract Refract Surg 2012;38:11:1911-7.



