High myopia is increasingly common, and more middle-aged people have unusually shaped nerves as a result. In the Bay Area, we now commonly see middle-aged Asian myopes without high eye pressure who have glaucomatous-like visual field defects discovered during screen-ing or by the patients themselves.
Here’s what you should be thinking about when managing a relatively young, highly myopic patient like this.
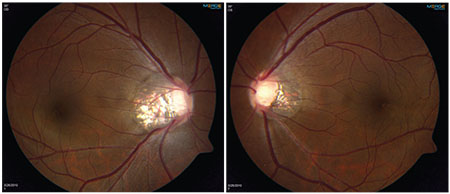 |
| A young myope with abnormal-looking discs but normal intraocular pressure, open angles and no signs of secondary causes of glaucoma. Both nerves are oval and tilted, with significant temporal peripapillary atrophy. |
Why It’s a Challenge
Diagnosing glaucoma in a patient who is highly myopic and has anomalous nerves and an abnormal visual field but normal pressures is a problem. That’s because we have three main ways to assess glaucoma: changes in the nerve, which can be measured by fundus photos and OCT imaging; changes in the visual fields indicating a progressive glaucomatous loss of visual function; and elevated intraocular pressure, which is currently our main modifiable risk factor for the disease.
Unfortunately, it can be difficult to identify the classic optic nerve changes for glaucoma in these highly myopic patients, and the fact that the pressure isn’t elevated is inconclusive, because glaucoma can still occur at “normal” pressures. Finding a worsening visual field in a specific pattern over time would certainly suggest that the patient has glaucoma; but by the time the patient’s visual fields have worsened, you’ve already lost the opportunity to prevent irreversible vision loss. Given that a correct diagnosis before visual field damage emerges is difficult at best, some myopes are probably being overtreated while others are being undertreated.
Matters are complicated even more by data showing that myopic refractive error is associated with glaucoma at a population level. Several large-population-based studies using different definitions of high myopia, including the Blue Mountains Eye Study, the Beijing Eye Study and the Los Angeles Latino Eye Study, have found that myopes are more likely to have glaucoma. The Blue Mountains Eye Study was criticized on the grounds that cataract-associated myopic shift was not taken into account; however, the Los Angeles Latino Eye study did correct for grade of cataract and axial length, and that data still demonstrated an association between myopia and glaucoma (in this study, myopia worse than -3 D). Of course, cross-sectional glaucoma diagnoses can be tricky, especially with myopic nerves, so it’s possible that some myopes were misdiagnosed as having glaucoma, for the challenging diagnostic reasons we’ve been discussing.
To help shed some light on how
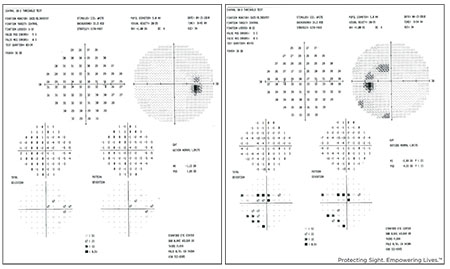 |
| Visual fields from the young myope shown above. The right eye had a thin retinal nerve fiber layer defect inferiorly, while the left eye had significant NFL loss inferiorly. |
A Case History
A 35-year-old Asian woman presented to our glaucoma clinic for evaluation about six years ago. She was a -8 D myope in both eyes and had 20/20 vision with contact lens correction. She had no prior laser or surgical interventions and wasn’t taking any ocular medications. She was aware that she had some family history of glaucoma, but didn’t know the details. Her pressures were normal, 18 mmHg in both eyes; her corneal thickness was slightly above average; she had open angles and a normal exam and didn’t show any signs of secondary causes of glaucoma. However, her optic nerves were very abnormal looking. (See images, above.) The nerves were both very oval and tilted, especially in the right eye, and there was significant temporal peripapillary atrophy in both. More concerning was a thin retinal nerve fiber layer defect in the right eye inferiorly. The left eye also had a large amount of nerve fiber layer loss, more inferiorly and nasally. The vessels were pushed far to the periphery, so the cup-to-disk ratio looked enlarged in both eyes, but it was difficult to assess the neuroretinal rim. Her optic nerves had features that could be attributable to either myopia or glaucoma.
Generally, when faced with a patient like this, you’d want to see the patient’s visual field defects. (See visual fields, below.) In this patient, you can see an abnormal field defect in the left eye; the right eye doesn’t have much of a defect. Given these fields in a myope without elevated eye pressure and no longitudinal data, I approached the patient as a glaucoma suspect and decided to proceed with preventive treatment. (I’ll review her six-year follow-up at the end of this article.)
To Treat or Not to Treat?
One way a definitive diagnosis can be confirmed is by detecting measurable change indicating a glaucomatous rate of progression—hopefully a small enough amount of change that the patient won’t have lost any visual function. However, in many cases simply accumulating enough risk factors and/or test results may justify the treatment choice. Thus, the more information you have, the better. With that in mind:
• See if OCT nerve fiber layer/ganglion cell analysis can provide additional information. It’s important to factor in your OCT findings when evaluating the amount of vision the patient has left. That’s true for two reasons: First, damage on OCT can precede visual field defects, so an OCT scan often may reveal damage that isn’t evident on the visual field. Second, a visual field defect could be an artifact; comparing it to OCT data should help to clarify whether the visual field defect makes anatomical sense. There are a number of caveats, however:
— Look for signs of an error in the OCT algorithm. Errors are more likely when scanning a highly myopic eye because of the structural alterations associated with high myopia. For example, in the scan shown to the right, the thickness data is cut off at the top (blue arrow), leading to signal loss and an algorithm error. The nerve fiber layer thickness map pattern can still be used, but the quantitative values won’t be accurate.
— Make sure that any significant defects you see on OCT correspond with defects on the visual field. For example, the patient we’ve been discussing has an inferior loss in the left eye and a superior arcuate bundle defect on the Humphrey 24-2 from the left visual field; there’s a little arc that comes off the blind spot in that eye. That defect matches the anatomic nerve fiber layer loss on the OCT.
— Beware of relying too much on the color codings of OCT results to indicate normal vs. abnormal. It’s best to compare each patient to his or her own baseline when looking for the smallest possible signs of change, rather than instrument analysis.
— Remember that normative data does not account for whether individuals have high myopia. That adds to the difficulty of following a high myope using OCT. For ex-ample, a 2012 study found that the superotemporal and inferotemporal RNFL bundles converge temporally with increasing myopia, which is associated with an increase in the area of abnormal RNFL measurement.2 The authors concluded that in-terpreting a spectral-domain OCT RNFL thickness map in myopic eyes requires careful consideration of the distribution pattern of the RNFL bundles.
Another (retrospective) study published in 2016 found that when myopes with open-angle glaucoma had inferiorly tilted discs, they were likely to progress faster than those with a temporally tilted disc or non-myopic open-angle glaucoma, and they were more likely to have inferior visual field loss.3 Again, this is not accounted for in normative data.
• Check the diurnal pressure. The appearance of normal IOP is one of the factors that makes these patients challenging to diagnose. Since the eye pressure reading is only a single measurement out of a continuum of IOP fluctuation, it’s best to obtain multiple readings at different times of day; schedule some follow-up visits in the morning and some in the afternoon.
• Try to find pre-existing clinical data. Part of the reason this decision can be challenging is that you’re working with data from a single point in time. Change caused by myopia is a result of expanding retinal defects and generally happens more slowly compared to change caused by glaucoma, so if there’s any way to obtain clinical data from previous years, you can easily determine whether the abnormality you’re seeing is long-standing or the result of worsening disease. If the old tests reveal the same defect with no change over years of time, you can be more confident that the abnormality is minimally progressive and choose to observe without therapy. Unfortunately, we often don’t have access to this kind of past data. However, that should become less of a problem with increasing electronic health record adoption.
• Focus on comparing the patient to himself or herself rather than the general population. Normative
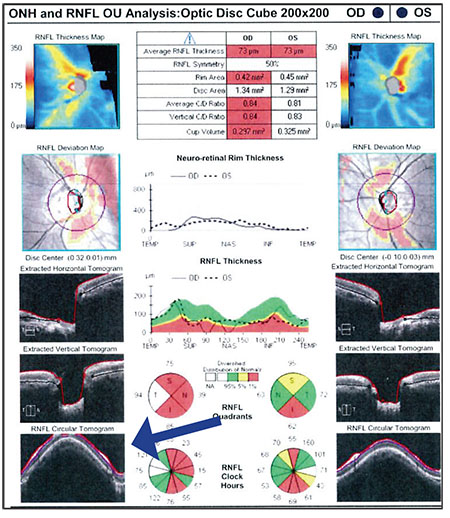 |
| It’s important to factor in OCT findings when judging how much vision the patient has left (a key factor in assessing the risk of waiting to see if progression occurs). However, look for signs of an error in the data, such as data being cut off (blue arrow). The nerve fiber layer thickness map can still be used, but the quantitative values won’t be accurate. |
For example, the images shown here (above, right) reveal what the optic nerves may look like in a pediatric patient who is extremely myopic. In this case, the patient’s left eye is -11 D; his right was emmetropic. You can see that the left nerve is noticeably different in appearance. If you were to encounter this patient at age 40, having never seen this photo before, you might be worried that the left eye was glaucomatous. However, if you had access to this image of the nerve at age 11, you might conclude that the nerve looked pretty much the same back then, and you’d realize the patient was born this way. That’s why this is so challenging; it can be hard to tell what’s an anatomic abnormality and what is the result of progressive change.
• Find out what the patient is willing to do. Given the choices of eye drops or laser therapy, some patients may choose preventative therapy even if their true risk is low; others may only want treatment once the diagnosis is more certain. That’s a discussion you need to have, to involve the patient in the treatment plan.
• Monitor the patient and look for change. If it’s not obvious whether the patient has glaucoma or just high myopia, you can choose to monitor the patient, looking for the earliest confirmed deterioration in test results over time—hopefully, small enough not to affect visual function in daily life. This may be an acceptable choice, depending on the age of the patient, the patient’s lifestyle and the amount of “reserve” tissue/vision that remains unaffected.
If you choose to monitor the patient, conducting serial testing using the same machine is key. You may want to do this on a more frequent basis at first, and then slowly space out the testing if no change is detected. In addition to following visual fields and OCT scans, be sure to monitor the optic nerve in comparison to baseline stereo disc photos; these have an advantage in that the technology doesn’t change over decades (unlike OCT and visual fields).
Consider the Risk
When you have no definitive evidence to guide your clinical choices, the amount of risk associated with the choice to observe rather than treat is a key consideration.
• Consider how much vision reserve the patient has. How much vision does the patient have left to lose? Is the patient already symptomatic? Usually someone with large functional deficits is at higher risk, especially if the patient still has many years to live. In particular, a visual field index <40 starts to indicate low reserve.
• Consider how close the existing damage is to fixation. Damage that’s already close to fixation puts the patient at greater risk, should the damage turn out to be caused by glaucoma. So, the closer the damage is to fixation, the more likely I am to treat, even if the patient has myopic retinal scars in the fovea that could also account for the field loss. (Usually the foveal threshold, if turned on during field testing, is depressed in early retinal disease relative to when it gets depressed in glaucoma.)
• If it appears to be glaucoma, consider how fast it’s likely to progress. This plays a role when deciding whether to advance to a minimally invasive glaucoma procedure versus filtering surgery. In young high myopes, we often wonder whether vascular risk factors play a role, leading us to seek out other treatable risk factors such as secondary causes, low blood pressure, migraines, sleep apnea, anemia, etc. One day there may also be a way to measure CSF pressure non-invasively.
Unfortunately, there’s no published data telling us what exactly to do in any given anomalous optic disc situation, so most of us will probably start by making the more conservative choice. If the patient has a large defect with little vision in reserve, I’m more likely to initiate treatment because the patient can’t risk any additional loss. On the other hand, if most of the eye testing still looks relatively normal—even with an abnormal-looking nerve—then I may choose to monitor for change to prove that glaucoma is the problem.
When You Might Want to Treat
If you’re leaning toward treating for glaucoma, consider these factors:
• If the patient has a paracentral defect and a disc hemorrhage not due to other causes, I see this as a high-risk sign and tend to treat the patient. In an article in the March 2017 edition of Review, “Redfining Primary Open-angle Glaucoma,” the author, Louis Pasquale, MD, dis-cusses different possible subtypes of open-angle disease, including a type he calls PC-OAG, or paracentral open-angle glaucoma. These patients have a set of distinctive signs: a para-central defect—more specifically, a triangular prelaminar neuronal defect within the cup that can be seen on OCT; a greater likelihood of disc hemorrhages; and a lower mean IOP than other glaucoma patients. They may also be younger than the average glaucoma patient, and are often described as normal-tension glaucoma patients.
As Dr. Pasquale notes—and my experience agrees—glaucoma pa-tients fitting this description tend to progress rapidly, and they need lower-than-normal target pressures with multiple therapies to minimize progression. These are high-risk patients, regardless of being young and having normal pressures.
• If the cornea is very thin, or the patient has had previous LASIK, err on the side of treating for glaucoma. In either of these situations, it’s more difficult to accurately monitor the patient’s true eye pressure. That increases the risk of misjudging the patient’s condition.
• Consider using a minimally invasive glaucoma surgery to treat a high myope. The well-known advantages of MIGS—being less risky for the patient and having fewer side effects compared to traditional filtering surgery, especially in high myopes—make a lot of sense when you’re not certain whether the ab-normality you’re seeing is truly the result of glaucoma. If the disease is present, this approach could be sufficient to change the course of the disease, with less chance of a deleterious effect on the patient, even if the abnormality was caused by high myopia rather than glaucoma.
• Avoid going straight to filtering surgery. In most cases, at the
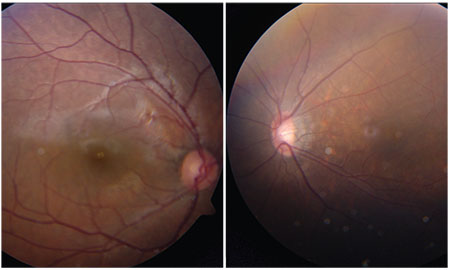 |
| An 11-year-old high myope (-11 D OS). If you saw this patient at age 40, you might think he had glaucoma. However, if you had access to this childhood picture, you would probably conclude that his eyes had always looked like this, altering your diagnosis. |
Moving Forward
The patient I described earlier initially agreed to use a single prostaglandin eye drop preventatively. While she was being treated, her intraocular pressure averaged be-tween 12 and 14 mmHg, down from 18 mmHg. But after two years, she became pregnant and needed to go off the medication, and she declined to undergo selective laser trabeculoplasty. From that point on, we followed her with structural and functional tests but no treatment. The glaucoma-progression analysis over that five-year period on both OCT and visual fields indicated that there was no change in either eye, even after stopping treatment, and her pressures stayed in the mid- to high-teens range. Thus, it appears that her defects were due to high myopia rather than glaucoma.
The main point to remember is that myopic degeneration can cause the optic nerve to have an abnormal appearance, as well as produce field defects that mimic glaucoma. So before you automatically treat a high myope who appears to have glaucoma but doesn’t have elevated eye pressure—especially before performing any kind of filtering surgery—take some of the steps described above. If the patient seems like a low-risk glaucoma suspect, it might make sense to follow the patient and see whether progression occurs. In higher-risk situations where treatment is called for, try lower-risk treatments initially such as drops, laser or MIGS surgeries. Only treat aggressively with filtering surgery if the patient has already lost substantial vision, or if you suspect the individual is potentially a fast progressor. REVIEW
Dr. Chang is an assistant professor of ophthalmology at the Byers Eye Institute, Stanford University School of Medicine. He’s a consultant for Alcon, Allergan, Santen, Iridex, Pfizer, Aerie, Unity and Kali Care, and has received grant funding from Carl Zeiss.
1. Ding X, Chang RT, et al. Visual field defect classification in the Zhongshan Ophthalmic Center-Brien Holden Vision Institute High Myopia Registry Study. Br J Ophthalmol. 2016;100:12:1697-1702.
2. Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: Interpreting the RNFL maps in healthy myopic eyes. Invest Ophthalmol Vis Sci 2012;53:11:7194-200.
3. Han JC, Lee EJ, et al. Visual field progression pattern associated with optic disc tilt morphology in myopic open-angle glaucoma. Am J Ophthalmol 2016;169:33-45.



