Remember when a full-thickness transplant was the only option for corneal disease? Not so today. Over the past decade, we’ve seen a shift toward disease-focused surgeries, and there are now several options to choose from when matching a procedure to a patient. The challenge is to pick the right one.
“We customize treatments now,” says Marjan Farid, MD, clinical professor of ophthalmology and director of both the cornea, cataract and refractive surgery; and the ocular surface disease programs at the UC Irvine School of Medicine. “If the disease is endothelial, then we move to endothelial keratoplasty. If it’s in the mid-layers of the cornea or stroma or anterior, we do more anterior lamellar keratoplasty. This shift toward disease-focused treatment has been a big advance in and of itself.”
Procedures such as DALK, DSAEK and DMEK have significantly advanced corneal surgery. In fact, Massimo Busin, MD, clinical director of the department of ophthalmology at Ospedali Privati Forlì, Italy, notes that “Lamellar procedures have largely replaced penetrating keratoplasty. In my practice, of the 564 transplantations performed last year, 55 percent were endothelial, 40 percent were anterior lamellar and less than 5 percent were penetrating keratoplasty.” In this article, we’ll cover the latest advances in corneal transplants and attempt to predict what the future has in store for these important, sight-saving procedures.
Disease-focused Approaches
Selecting a procedure goes beyond determining which corneal layers are affected. While standard-of-care routes are standard for a reason, you don’t have to follow these guidelines to the letter, say surgeons such as Kathryn Colby, MD, PhD, chair of the department of ophthalmology and visual science at the University of Chicago Medicine and Biological Sciences. “You want to ask yourself where the pathology is and what the pathology is,” Dr. Colby says. “Your standard of care for Fuchs’ is DMEK, and sometimes DSO, but this is a general recommendation. Certain Fuchs’ patients may be better served with a DSAEK or even a full-thickness transplant. It comes down to surgical experience and judgment.”
Dr. Farid explains how she decides, in general, which corneal procedure is best-suited for a patient. “If a patient comes in with a corneal disease, you first isolate where the disease is,” she explains. “Is this an endothelial disease like Fuchs’ dystrophy or is this like keratoconus, where it involves the entire stroma and a misshapen cornea? If it’s endothelial disease, I go down the road of endothelial keratoplasty. If it’s a simple Fuchs’ dystrophy and they have good visibility into the eye then the gold standard has become DMEK. However, if there’s a lot of complexity in the eye or if the patient’s visibility through their cornea and edema is significant, I may choose a DSAEK procedure over DMEK because it’s a little more predictable and easier to do through a hazy cornea. If it’s a keratoconus eye then I lean more toward a DALK and I always use a femtosecond laser to do cuts to get the best refractive outcomes.”
The range of DMEK’s applications has also broadened. “When DMEK was first being popularized, it was suggested that it was best for otherwise healthy eyes with FECD,” says Brandon Baartman, MD, in practice at the Vance Thompson Vision in Omaha, Nebraska. Many surgeons, seeing the benefits to the patient, have since pushed these initial, arbitrary boundaries to bring DMEK to more complex scenarios, such as in patients with significant PBK, prior failed EK or in the setting of prior glaucoma filtering or shunting procedures. Many of the cornea talks at national meetings are now shifting from a focus on how to perform the routine DMEK to focusing on some of the ways it can be used successfully in unique scenarios. Certain techniques such as a DMEK ‘pull-through’ using insertion devices have helped navigate challenging cases.”
The Pull-Through Technique
To perform the pull-through technique, a surgeon places a graft by pulling it through a clear corneal incision on a substrate, such a soft contact lens, corneal lenticule or an insertion device. The contact lens-assisted pull-through technique is one new approach for inserting tri-folded DMEK grafts.1 According to a 2016 prospective, noncomparative, interventional case series, including 42 eyes with Fuchs’ with or without cataract, this pull-through technique reduced surgical trauma to donor cells and facilitated spontaneous unfolding, which minimized surgical time.1 Dr. Busin, the lead author on the study, explains his current technique for DMEK graft preparation and insertion. He uses pre-marked, pre-stripped donor tissue stained with Trypan blue and punched with a Barron Donor Cornea Punch. “Using a dedicated anatomic microincision forceps (Moria SA, Antony, France), the graft is tri-folded endothelium-in, transferred via a sterile soft contact lens into the groove of an IOL cartridge that is filled with BSS and sealed with a silicone plug,” he says. “Then, using the same microincision forceps, the tri-folded graft is delivered bimanually into the anterior chamber under continuous irrigation from a dedicated AC maintainer with a lateral 0.5-mm port. The tri-folded graft spontaneously unfolds endothelium-out and air is injected for graft tamponade.”
Dr. Busin says he’s currently developing a dedicated system for the pull-through delivery of tri-folded DMEK grafts that includes “a fixed-depth corneal donor punch, a sliding glide (which replaces the sterile soft contact lens) and a glass cartridge (which replaces the IOL cartridge).” He hopes that “This new system will streamline our process from graft preparation to insertion and perhaps allow more beginning surgeons to be more comfortable with adopting DMEK.”
Thinner Tissue
Another innovation in corneal transplant procedures, particularly endothelial keratoplasty, is the shift toward thinner and thinner tissue grafts. “Ultra-thin and nano-thin grafts result in better visual acuity and have a lower risk of rejection, leading to better graft survival,” says Dr. Baartman. “DMEK is one of the latest iterations of tissue transplantation, representing a 1:1 tissue replacement,” he says.
“Ten or 11 years ago when we started doing endothelial transplants, the tissue we were implanting was quite thick,” adds Dr. Farid. “We were doing deep lamellar endothelial keratoplasty, or DLEK, which involves a very thick piece of posterior stroma and endothelium. This treated the disease, but the visual outcomes weren’t great because we were adding significant tissue to the eye. Now we’ve moved toward Descemet’s stripping endothelial keratoplasty and then ultra-thin and even nano-thin DSEK, which use thinner and thinner tissue. We’re seeing that the thinner the tissue, the better and faster the visual recovery is. This also means intraoperative manipulation is more challenging,” she adds. “However, with newer insertion instruments such as the Endoserter (CorneaGen), it’s getting easier and more predictable to do thinner DSEK.”
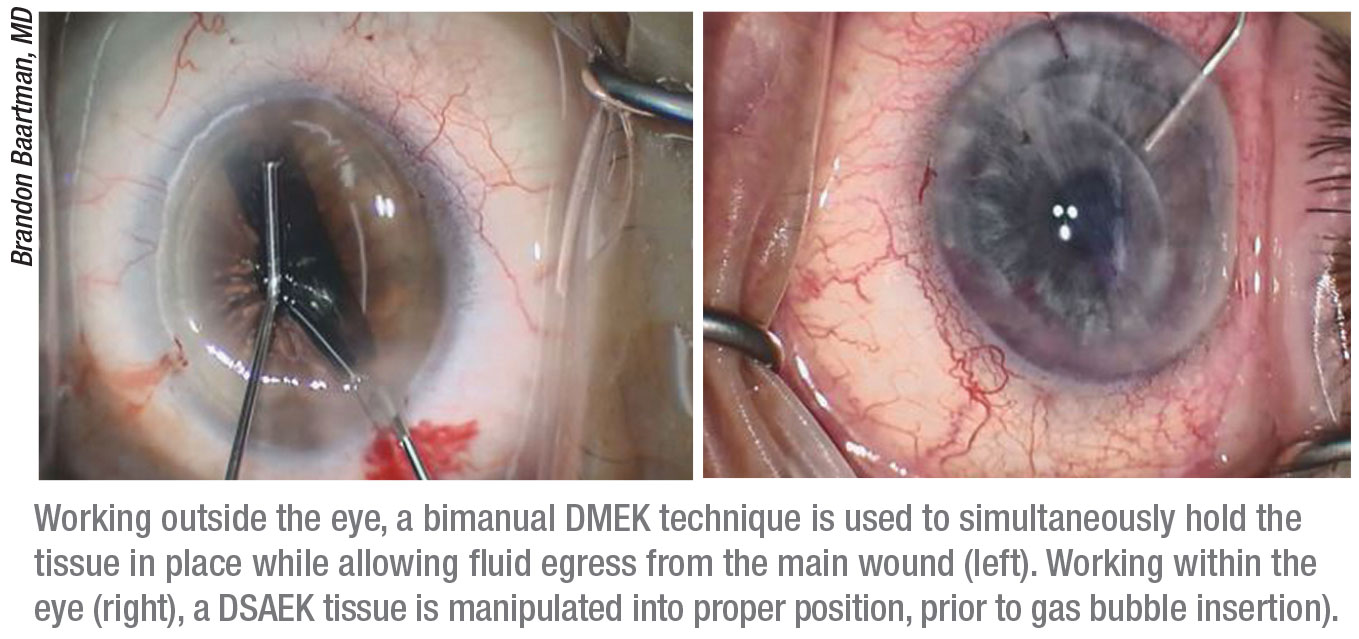 |
The Role of Eye Banks
Dr. Colby and her research team members were among the early adopters of preloaded DSAEK tissue from eye banks. “The eye banks have made tremendous advances for us,” says Dr. Colby. When the Lions Eye Institute for Transplant and Research in Tampa began preloading DSAEK tissue to ship as a test run, Dr. Colby ordered some and conducted a trial that found donor graft tissue preloaded by an eye bank could be successfully used for endothelial keratoplasty.2 She and her research partner noted that the eye bank’s pre-processing and preloading of tissue reduced intraoperative tissue manipulation.
“Back in 2006 when eye banks began precutting tissue, we went from doing about 5,000 EKs a year to about 15,000,” says Dr. Colby. “I think eye banks may be a tipping point for endothelial keratoplasty in the United States.”
Dr. Colby explains that there was more risk involved when surgeons had to take full responsibility for cutting tissue. “If you messed up, you couldn’t transplant it, and your hospital had to assume the cost of the tissue—which is not inexpensive,” she says. “Eye banks take that risk away from surgeons. Most surgeons might do four or six DMEKs in a month as opposed to someone whose job it is to prepare tissue every day.”
“In the setting of endothelial transplantation, any amount of tissue preparation which the eye bank can perform for the surgeon speeds us up,” Dr. Baartman notes. “We saw this with DSEK automation and we’re seeing the same thing in DMEK, with pre-loaded tissue. When I trained in DMEK, the tissue staining, punching, preparation and loading all happened in an OR prior to the patient’s arriving. With preloaded DMEK, the tissue and patient arrive in the OR at the same time, and minimal surgeon time is required for in-OR preparation. I believe the shifting of this work to those who provide tissue to surgeons lends itself to optimization of techniques and processes.”
Dr. Baartman says two critical metrics for corneal transplant success have improved with the use of thinner donor tissue—postoperative BCVA and rejection rate. “With DMEK, it’s not uncommon for us to see BCVA of 20/20 in patients as early as one to two weeks after transplant. In regard to rejection, I typically cite a 1-to 2-percent chance of tissue rejection for DMEK versus DSAEK, the latter of which I cite to be around 5 percent. It’s important to remember that this can also be impacted by the indication for transplantation,” he adds. “Fuchs’ patients typically fare better than those patients with BK, regardless of technique used.”
Dr. Busin says using pre-stripped, pre-marked tissue for DMEK allows him to “evaluate the graft and ensure proper orientation of the tri-fold during implantation. Tissue is S-stamped or F-stamped on the stromal side to help the surgeon orient the tissue correctly. Incorrectly-positioned grafts can lead to graft failure. For DSAEK and DALK, I prepare the grafts with the microkeratome based on pachymetry.”
New EK, New Challenges
Experts agree that DMEK is the gold standard for endothelial keratoplasty. “Recent studies have suggested that DMEK is better than DSAEK in terms of final visual outcomes,3,4 but there’s still room for DSAEK because not every case that needs an endothelial keratoplasty would be appropriate for DMEK,” says Dr. Colby.
Dr. Baartman says one of DMEK’s strengths is its minimization of tissue usage. “We’ve known for many years that the less corneal tissue you transplant, the less risk of rejection (e.g., EK versus PKP),” Dr. Baartman says. “Data is suggesting the same thing when looking at DMEK versus DSAEK. Overall graft survival is better with DMEK as compared to DSAEK, as are visual acuity outcomes. Even within the subcategory of DSAEK, ultra-thin and nano-thin techniques are being used to transplant the least amount of stromal tissue to capitalize on this knowledge.”
With thinner tissue and no stroma to lend form, however, wispy DMEK grafts can be a challenge to manipulate. “Tissue curls endothelial-side out without the stroma to give it form,” explains Dr. Colby. “That means the manipulation has to be done taking into account that any time you touch the tissue, you’re touching endothelium. In contrast, DSAEK unfolds much more easily because it’s got substance—maybe 40 to 120 µm of stroma. It’s easiest to get DMEK tissue to unroll in a shallow chamber. In DSAEK, chamber depth isn’t as much of an issue, and in fact, you may want a slightly deeper chamber because the tissue takes up more space.”
“Another challenge with thinner grafts is the higher rebubbling rate [to facilitate attachment and avoid postop detachment],” Dr. Baartman says. Factors influencing rebubble rates in DMEK still need more study, but one group found that higher endothelial densities and well-centered Descemet’s grafts may lead to fewer complications, and that a surgeon’s decision to remove or leave a gas bubble doesn’t affect whether or not a patient will need a rebubble.5
Femtosecond Lasers
“In the realm of deep anterior lamellar keratoplasty and full-thickness transplants, a big area of advancement is the use of the femtosecond laser for creating customized trephination wounds for full-thickness keratoplasty and DALK,” says Dr. Farid. “We’ve seen that with these customized femtosecond laser-enabled wounds, eyes have faster wound healing because with more surface area, they have more regular sealing with less irregular astigmatism. Sutures also come out faster and visual recovery takes less time.
“The original laser used was the Intralase (now iFS by Johnson & Johnson Vision),” she continues. “However, newer, faster lasers such as the VisuMax (Carl Zeiss) are also showing promise in creating smooth and predictable cuts.” Other femtosecond lasers for keratoplasty include the Victus (Bausch + Lomb), Femto LDV Z8 (Ziemer), WaveLight FS200 (Alcon) and FEMTEC (Bausch + Lomb and 20/10 Perfect Vision).
Dr. Farid describes her trephination wound patterns as zig-zags or mushroom-shapes. These patterns increase the surface area of the graft and facilitate better wound alignment.6 Her current research in femtosecond lasers aims at improving surgeons’ ability to make deep corneal cuts for a more predictable DALK.7
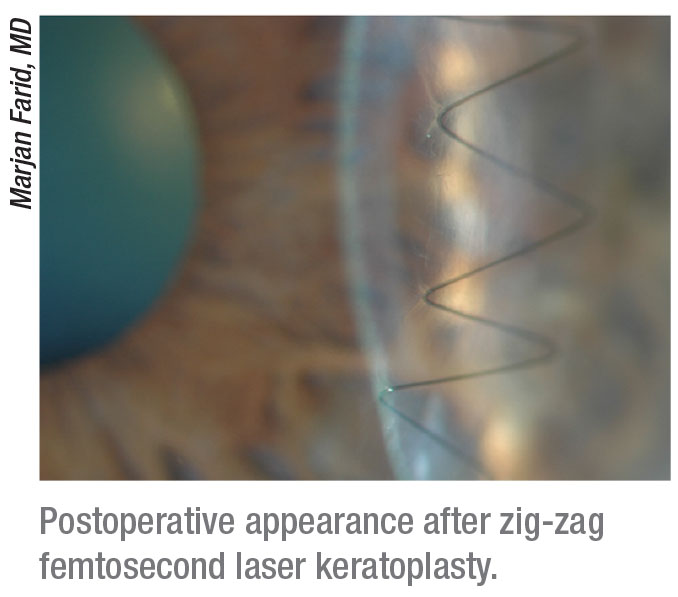 |
“Manual trephination of the cornea is usually a straight up-and-down wound, like a butt joint in carpentry where it’s just two straight incisions coming together,” she explains. “But with a butt joint, you can have vertical and torsional misalignment of the tissue. That’s how current conventional full-thickness transplants are done. The femtosecond laser creates more of a tongue and groove joint-type incision. This zig-zag incision creates a nice anterior alignment of the host and donor tissue. Better fit and less irregularity of the tissue mean faster healing.”8
Though PK has been supplanted as the go-to procedure, there’s still a place for it, argues Dr. Farid. “In the university setting, we see a lot of infections of the cornea that are full-thickness,” she continues. “Full-thickness corneal transplants aren’t going away—there are still a lot of scars that go through all the layers of the cornea and require full-thickness transplants. We’re able to use femtosecond laser technology for full-thickness transplants in noninfected cases as well to optimize visual outcomes.”
Descemet’s Stripping Only
A newer technique that doesn’t involve transplant of donor tissue has been on the rise. “Descemetorhexis Without Endothelial Keratoplasty, or Descemet’s Stripping Only, involves the removal of central, visually-significant guttae without inserting new tissue,” says Dr. Baartman. “I consider these techniques more like ‘endothelial rejuvenation.’ ”
DSO requires some healthy peripheral endothelial cells to migrate across the posterior cornea to the center, so it’s suitable only for mild to moderate cases of Fuchs’ where the diseased endothelial cells are right in the central 5 mm of the cornea. “Oftentimes, a rho kinase inhibitor, or ROCK inhibitor, is used to help the peripheral cells of the endothelium migrate into the center to clear the central corneal edema,” says Dr. Farid.
Beginning in early 2020, a large, multicenter clinical trial is taking place to study DSO for Fuchs’. Dr. Colby is the principal investigator, and has been a major leader in DSO research. “In the days of full-thickness transplantation, we used to counsel patients that about maybe one out of three or one out of four would have a rejection episode at some point during the life of the transplant,” Dr. Colby explains. “With DSAEK, it’s much less—less than 10 percent—and with DMEK, if you stay on topical steroids, it’s less than 1 percent. With DSO, however, there’s no risk of rejection.
“There’s no transplant, unlike with traditional EK,” Dr. Colby continues. “You remove the bad cells, like the first step of endothelial keratoplasty, but then you’re relying on the patient’s own endothelium to repopulate the central cornea. You first mark the central guttae and make an incision in the cornea. Then you go in and remove the Descemet’s membrane with the guttae on it using a variety of techniques. Most recently, there’s a special forceps designed for this.”
Dr. Baartman uses blunt-tip DSO Gorovoy forceps from Moria Surgical. He describes them as similar to capsulorhexis forceps, except the tips are pointed upwards like an inverted Utrata. “This allows a rhexis-like removal of the membrane, and it’s actually easier and faster to remove,” he says. “The forceps were designed for more precise removal of the Descemet’s membrane in the setting of DSO procedures.”
DSO is indicated for Fuchs’ dystrophy only. If a patient presents with guttae over the entire cornea, DSO will not work for her, and she’ll need an endothelial keratoplasty.
“DSO is growing in popularity,” explains Dr. Colby. “In 2015 or 2016, people weren’t sure of the position it might hold within our transplant armamentarium. But now there are many more people doing DSO.”
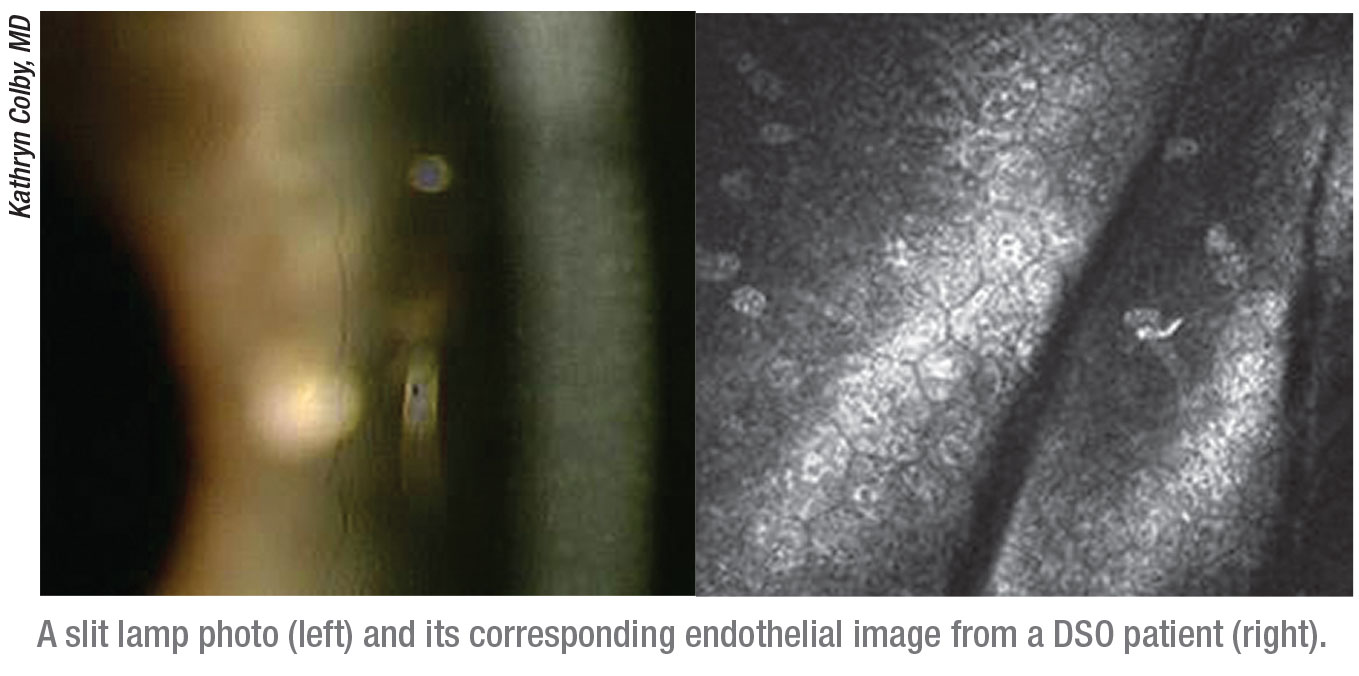 |
Other Non-graft Options
“In terms of advances in medication, I think the ROCK inhibitor is very exciting,” says Dr. Farid. “We’re looking forward to testing that out in this 2020 trial to see if it really makes a difference in terms of helping endothelial migration and stability, so that we can do more DSO instead of having to transplant tissue.”
“The recent literature suggests that the use of ROCK inhibitors after DSO and DMEK may increase final cell count,” adds Dr. Colby.
Dr. Colby and Dr. Farid say that researchers in Japan are paving the way for ROCK inhibitor research. “Maybe at this point four dozen patients have been injected with cultured endothelial cells, supplemented with a ROCK inhibitor,” says Dr. Colby. “The theoretical advantage of cells is that you can take one donor cornea and potentially make enough cells to transplant to 40 or 60 or even 100 patients. That kind of cell replenishment surgery can be done for all forms of endothelial dysfunction, whereas Descemet’s stripping works for only Fuchs’ where there’s healthy endothelium in the periphery. Further advances are also under way with potential ex vivo expanded endothelial cells that can be injected into the eye.”
Surgeons agree that injectable endothelial cells may be the next big wave in approaching endothelial disease. The goal would be for patients to receive an injection of endothelial cells, and then be positioned face-down for a few hours. “The next step is bringing the technology to the United States,” says Dr. Colby.
Noriko Koizumi, MD, PhD, professor of biomedical engineering at Doshisha University, Kyoto, Japan, and her team worked on developing cultivated corneal endothelial cell sheets for transplantation. “Human corneal endothelial cells are very difficult to culture,” she says. “We tried various compounds, and finally found the Y-27632 ROCK inhibitor stimulates the proliferation of human CECs. We also found that Y-27632 promotes the cell adhesion to the substrate. This finding led us to develop cell-injection therapy. We’re working on cell-injection therapy with ROCK inhibitors for advanced-stage FECD and pseudophakic bullous keratopathy.”9,10,11
In 2013, her team initiated a clinical trial to test to the effectiveness of cultured human CECs injected into the anterior chamber of patients with corneal endothelial decompensation. “The results were surprising and very impressive to me,” says Dr. Koizumi. “Most of our patients had clear corneas and recovered good vision, almost to 20/20.”
One exciting finding came with hope provided by ROCK inhibitors. “Human CECs had been considered non-regenerative, but the ROCK inhibitor results told us we could somehow proliferate corneal endothelial cells in vitro and in vivo,” says Dr. Koizumi. At the time of their 2017 review of ROCK inhibitors for treating corneal endothelial dysfunction,10 Dr. Koizumi and her team had treated 31 patients with cell-injection therapy. “This may be a paradigm shift in the treatment for corneal endothelial diseases,” she says.
Compared to tissue-engineered corneal endothelial cell sheets, Dr. Koizumi says cell-injection is far easier to deliver. The next step for her team is development of a cell-therapy product in collaboration with a startup company. “It’s for early-stage Fuchs’ patients,” she explains. “We’re developing an eyedrop (not a ROCK inhibitor) to prevent the apoptosis of corneal endothelial cells.”
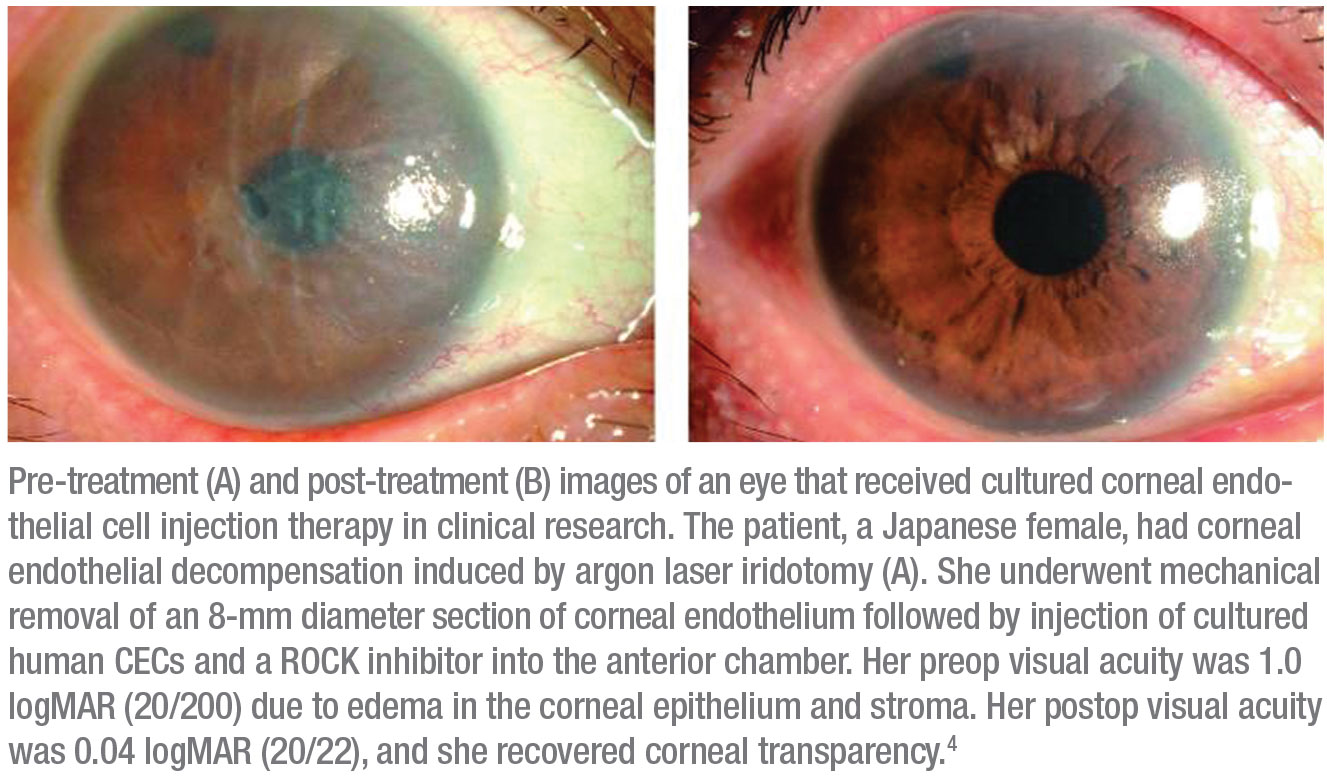 |
Outside Challenges
Aside from the challenges of manipulating thinner tissue, one of the biggest challenges surgeons face with corneal procedures comes from outside the eye—their patients. Today, patient demands concerning refractive outcomes have increased. “Patients don’t want to wear glasses and really want improved refractive outcomes,” says Dr. Farid. “It’s not enough to have a clear graft and put patients into hard contact lenses. They want to be free of rigid contact lenses for the rest of their lives or they want to get low-prescription glasses or have complete spectacle independence. So, we really need to deliver not only clear grafts, but better quality of vision.”
All this patient demand has challenged surgeons to be more innovative. Dr. Farid points out that now, “as a result, patients have fewer HOAs, less irregularity and are better able to get low—or no—spectacle correction.”
Pearls
With so many procedures to choose from and some new advances to be on the lookout for, here are some tips to keep in mind.
• Counsel patients carefully. “Procedural success is predicated on the health and proper functioning of the new tissue,” Dr. Baartman says. “Sometimes this tissue needs a little extra care, like additional air bubbles or steroids. It’s important that patients understand the process so they don’t feel as if their procedure is going wrong or that the extra work is a complication.”
• Choose the right procedure for each patient. Matching the appropriate technique to the appropriate patient is key, says Dr. Colby. “You have to know all the benefits and disadvantages of the entire armamentarium. There’s no one-size-fits-all.”
• Master the different surgical techniques and use them wisely. “A well-executed DSAEK will yield better outcomes than a poorly-performed DMEK,” says Dr. Busin.
• Choose straightforward cases if you’re just starting out. Dr. Farid says that when she’s teaching new surgeons how to do DMEK, she advises them to select eyes that are clear, straightforward and have good visibility. “You don’t want to be doing your first 20 DMEKs on eyes with poor visibility, tube shunts or other glaucoma-related hardware,” she says. “DSAEK or ultra-thin DSAEK is better for those complicated eyes. DSAEK is a more predictable procedure than DMEK, and the tissue is a little thicker, so it’s easier to handle.”
The Corneal Crystal Ball
“I predict that with techniques like cell culture, stem cell engineering, and other tissue rejuvenating advances, we’ll likely see Descemet’s membrane left alone in many eyes with endothelial cell loss, and we’ll replace or enhance only diseased cells,” says Dr. Baartman. “Less invasive techniques may lead to earlier intervention, with replacement occurring at early guttata development. I also believe we’ll continue to see less bullous keratopathy with improved phacoemulsification efficiency and techniques.”
Dr. Colby predicts that “we’ll figure out early which patients will do well with DSO and ROCK inhibitors so that we’ll be able to offer something to Fuchs’ patients before they would need a transplant. Many doctors tell their patients to wait until their diseases are ‘bad enough’ for a transplant. If a patient’s not having symptoms, I wouldn’t recommend surgery, but I could see offering DSO to someone just beginning to have morning blurring symptoms or vision problems if we develop a solid way of predicting successful outcomes for DSO.
“I also think we’ll see progress in cultured cell therapy,” she says. “I’m imagining a future where you can have packaged endothelial cells available for order.” Dr. Farid shares this hope. “If we could inject endothelial cells into the cornea and have the patient lie face-down for a few hours, we could cure them,” she says.
The changes in corneal transplantation over the past 20 years have been remarkable, says Dr. Colby. “Back in the 90s, we basically waited for patients to be blind before we did full-thickness transplants on Fuchs’ patients because of the long visual recovery and the many problems associated with full-thickness transplants,” she says. “Fast forward 20 years, and we can strip off the endothelium and they do fine. My first patient is six years out; he’s still 20/20 and very happy.” REVIEW
Drs. Colby and Busin have no relevant financial relationships to disclose. Dr. Koizumi has financial relationships with Senju Pharmaceutical and ActualEyes. Dr. Farid reports no relevant financial relationships, but notes that she’s a consultant for Johnson & Johnson Vision and uses the company’s femtosecond lasers in her research.
1. Busin M, Leon P, Scorcia V, Ponzin D. Contact lens-assisted pull-through technique for delivery of tri-folded (endothelium-in) DMEK grafts minimizes surgical time and cell loss. Ophthalmology 2016;123:476-83.
2. Palioura S and Colby K. Outcomes of Descemet stripping endothelial keratoplasty using eye bank-prepared preloaded grafts. Cornea 2017;36:21-25.
3. Singh A, Zarei-Ghanavati M, Avadhanam V, Liu C. Systemic review and meta-analysis of clinical outcomes of Descemet membrane endothelial keratoplasty versus Descemet stripping endothelial keratoplasty/Descemet stripping automated endothelial keratoplasty. Cornea 2017;36:11:1437-1443.
4. Durrani AF, Faith SC, Jhanji V. Ultrathin Descemet stripping automated endothelial keratoplasty. Curr Opin Ophthalmol 2019;30:4:264-270.
5. Mechels KB, Greenwood M, Sudhagoni R, et al. Influences on rebubble rate in Descemet’s membrane endothelial keratoplasty. Clin Ophthalmol 2017;22:2139-2144.
6. Farid M, Kim M, Steinert RF. Results of penetrating keratoplasty performed with a femtosecond laser zigzag incision initial report. Ophthalmology 2007;114:12:2208-2212.
7. Farid M, Steinert RF. Deep anterior lamellar keratoplasty performed with the femtosecond laser zigzag incision for the treatment of stromal corneal pathology and ectatic disease. J Cataract Refract Surg 2009;35:5:809-813.
8. Farid M, Steinert RF, Gaster R, et al. Comparison of penetrating keratoplasty performed with a femtosecond laser zig-zag incision versus conventional blade trephination. Ophthalmology 2009;116:9:1638-1643.
9. Okumura N, Kinoshita S, Koizumi N. Application of rho kinase inhibitors for the treatment of corneal endothelial diseases. J Ophthalmol 2017;2646904.
10. Okumura N, Kinoshita S, Koizumi N. The role of rho kinase inhibitors in corneal endothelial dysfunction. Curr Pharma Design 2017;23:660-6.
11. Kinoshita S, Koizumi N, Ueno M, et al. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. N Engl J Med 2018;378:995-1003.



