Descemet's stripping endothelial keratoplasty has been a revolutionary change in the treatment of endothelial disorders, avoiding the need for the more invasive penetrating keratoplasty. However, it's not without its problems, one of which is the trauma that can befall the delicate endothelial graft when it's manipulated by forceps. As a possible solution, surgeons and companies are developing injectors that may be able to insert the graft with less trauma to the endothelial cells. Here are three innovative designs that may hit the market in late 2008 or early 2009.
• Keramed Endoshield. The Endoshield uses a cartridge approach to insert the graft into the anterior chamber. However, the cartridge isn't like that used to insert an intraocular lens.
"The patent-pending cartridge causes the graft to curl in on itself," explains the Endoshield's inventor and Keramed President Yichieh Shiuey, MD. "The resulting column strength allows you to push against it. An analogy would be trying to push a thin piece of paper by just pushing on one end. It would be difficult because it's so floppy and thin. But, if you roll up that piece of paper, it has column strength that you're able to push against to get it to move.
"The problem with rolling up a DSEK graft like a newspaper, though, is if you just roll it up, you damage the endothelium," Dr. Shiuey continues. "We came up with the idea that you have to curl the tissue up slightly on both sides so there's no endothelial touch when it's rolled." He says Keramed is currently in the process of making a cartridge that will curl the graft inward so it will fit through a smaller wound than the 5-mm incision that's currently used in DSEK, while avoiding endothelial touch. "For a given size graft, the Keramed injector can go through a smaller incision than would be possible if you just curled both ends of the circular graft until they touched," says Dr. Shiuey. "We've done insertions through 3-mm incisions, and they work all right, but we'd suggest beginning surgeons start with an incision around 3.5 mm and move on to 3-mm wounds when they're comfortable with the process."
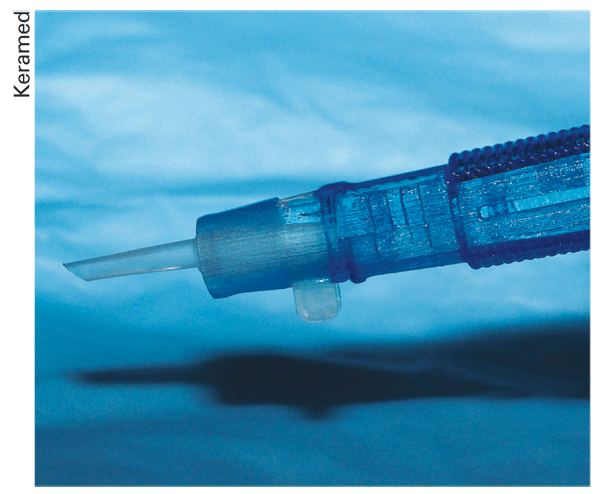
The Keramed Endoshield DSEK injector uses a propietary method to fold the graft small enough to fit through a 3.5-mm incision.
Currently there are no endothelial cell-count studies of the Endoshield vs. traditional forceps implantation. Though such a study is planned, at this point the only comparison Keramed has is a qualitative study using vital staining, performed by Sonia Yoo, MD, of Bascom Palmer. In it, Dr. Yoo stained five cadaver grafts that came out of the injector and found minimal endothelial damage, says Dr. Shiuey.
"Right now, the focus of our company is to be able to get something usable into the hands of surgeons," says Dr. Shiuey. "So, we're completing the process of manufacturing and then, before we actually give it to anyone, we want to do cadaver eye studies to document how it's performing, and then publish that data." He hopes to get 510(k) approval for the device and make it available in the
• Rhein Medical/LensTec Harvey-Steinert Injector. Like the Keramed Endoshield, the Harvey-Steinert Injector isn't yet 510(k) approved. To deliver the graft into the recipient's eye, this device uses a reusable, stainless steel, single-handed handpiece and a disposable carrier that acts like a sled for the graft to ride on. "Basically, the sled is attached to a syringe-like piston that allows the cornea to be loaded right near the tip, retracted into the shooter and then injected through the incision to allow delivery of the donor graft," explains the device's co-inventor, Thomas Harvey, MD, of Eau Claire, Wis. "So, it's like a syringe, and very similar to the IOL injectors that most surgeons are familiar with.
"The advantage of a carrier-assisted injection is that the tissue is never compressed," Dr. Harvey adds. "It effectively rolls in the lumen of the injector and carrier, avoiding endothelial touch."
No endothelial cell counting has been performed with the Harvey-Steinert device yet. However, like the Endoshield, qualitative research has been done. "In preliminary animal testing, there's no touch of endothelial cells," explains Dr. Harvey. "Though no cell counts have been performed, the actual gross observation studies have been successful. In a month [August] we're going to do an ex vivo lab study with cadaver corneas, putting them in a model to show that the endothelial cell loss is less than our limit of 20 percent." This would be an improvement over insertion with forceps, since a prospective study of DSEK using forceps for graft insertion found cell loss at a rate of 34 percent at six months.1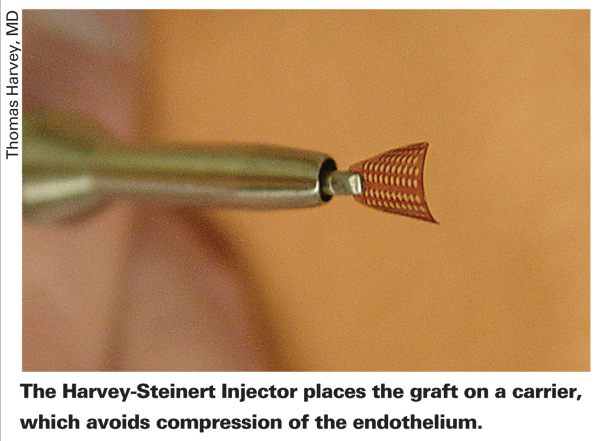
"I really believe the future of donor insertion for posterior lamellar grafting will use an injection system so we can have a more reproducible and safer means to deliver the necessary tissue," says Dr. Harvey. "No matter how careful one is while using forceps, the point of a forceps is to squeeze, and that's not what we want to do to a donor graft."
• Al-Ghoul Vacuum-assisted Injector. This device takes the novel approach of using the existing vacuum technology of a phaco machine and using it to hold the graft.
"We tried other ways of transporting the tissue with the least amount of damage, and found that we might be able to achieve the least amount of corneal manipulation and still safely deliver the tissue using the vacuum technology in a phaco platform," says the device's inventor Ahmed Al-Ghoul, MD, of the University of Pittsburgh Medical Center. He also receives support from the biomedical engineering company Emsiom. "Basically, you have an injector that has an acrylic cartridge, and a tip that's mobile—you can move it in and out of the cartridge. You place the corneal tissue on the metallic tip, stromal side down, and when the vacuum has risen to the point of complete occlusion and the tissue is stabilized, you retract the metallic tip into the cartridge and rotate the injector in your hand so the vacuum ports are facing downward. When you're ready to enter the cornea, you make the clear cornea wound, and insert the acrylic cartridge. Then, once you have the cartridge in the opening, you extend the metallic tip again, but this time it's got the corneal tissue on it." The injector also has an irrigation system, supplied at a point distal from the vacuum occlusion site, that maintains the stability of the anterior chamber as the tissue is inserted. The injector currently can be used comfortably with a 4.5-mm incision, Dr. Al-Ghoul says, though use of a 4-mm incision is possible.
To release the tissue once the tip is extended in the recipient's eye, the surgeon stops the vacuum and presses reverse vacuum on the foot pedal, which sends fluid out through the vacuum ports, releasing the donor tissue into the anterior chamber, endothelial side down. The irrigation continues during this step. The surgeon retracts the tip and removes the injector. "You'll see the cornea sitting in the anterior chamber," says Dr. Al-Ghoul. "You then use an air syringe to lift the donor tissue up onto the recipient cornea."
Dr. Al-Ghoul and his colleagues are currently tweaking the injector's ability to handle larger diameter tissues. The largest they can currently insert is 8 mm. "We'd like to increase it to 8.5 or possibly 9 mm," he says.
In terms of potential availability, Dr. Al-Ghoul will perform endothelial cell survival studies in two to three months, and hopes to achieve 510(k) approval for the device sometime in November.
1. Terry MA, Chen ES, Shamie N, et al. Endothelial cell loss after Descemet"s stripping endothelial keratoplasty in a large prospective series. Ophthalmology 2008;115:3:488-496.