The diagnosis of choroidal melanoma is clinical and supported by ancillary imaging findings. Recent advances in multimodal imaging allow for more accurate tumor characterization and a sophisticated approach to monitoring tumor response to therapy. Fundus photography, ultrasonography, fundus autofluorescence, angiography
Fundus Photography
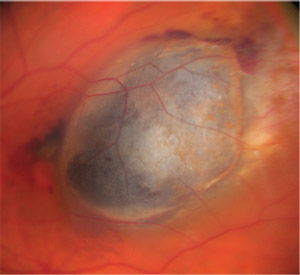 |
| Figure 1. Choroidal melanoma in the macula captured with standard color fundus photography documents typical features: a dome-shaped pigmented choroidal mass with overlying orange pigment, the absence of drusen and the presence of subretinal fluid. |
Since the development of the fundus camera in the early 1900s, progress in the field of digital imaging has revolutionized the ability to capture clinical observations. The early fundus camera introduced by Zeiss and
More recently developed non-mydriatic, ultra-widefield imaging systems capture up to 200 degrees of the fundus in a single image. For peripherally located tumors, these systems allow for more complete documentation of tumor margins and associated features such as exudative retinal detachment, particularly when multiple clock hours of the fundus are involved (Figure 2). The ability to accurately photograph tumors located anterior to the equator at regular time intervals following primary therapy is important for assessing response to therapy and monitoring for recurrence at tumor margins.3 While advantageous in this regard,
Fundus Autofluorescence
FAF can be performed using both standard and ultra-widefield imaging systems. This modality can be particularly helpful in confirming the presence of overlying lipofuscin, a frequent clinical feature of choroidal melanoma. With FAF, hyper-autofluorescence results when lipofuscin is exposed to intense blue light with a wavelength of 488 nm.4 Lipofuscin accumulates in the retinal pigment epithelium and in macrophages, but its appearance is variable depending upon the pigmentation of the underlying lesion. For highly melanocytic tumors, lipofuscin will characteristically be bright orange. For more lightly pigmented or amelanotic choroidal melanoma, lipofuscin may appear “ruddy” brown. Other benign tumors, such as circumscribed choroidal hemangioma, can also have overlying lipofuscin, but its presence may be nearly undetectable by ophthalmoscopy. FAF is useful for identifying lipofuscin, and although not pathognomonic for choroidal melanoma, its presence is supportive of the diagnosis.5
Angiography
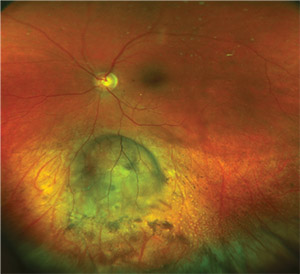 |
| Figure 2. Ultra-widefield color fundus photography completely captures a choroidal melanoma in which the anterior margin is overlying the equator. Following treatment with I-125 plaque brachytherapy, The tumor demonstrates regression with a ring of surrounding chorioretinal atrophy. |
Fluorescein angiography is available in both standard format and in
Optical Coherence Tomography
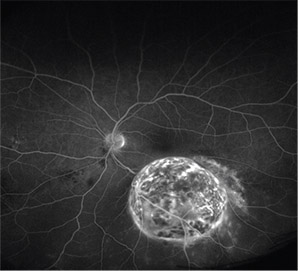 |
| Figure 3. Ultra-widefield fluorescein |
Since its introduction, OCT has become widespread in ophthalmic practice.6 Spectral-domain OCT has essentially replaced conventional
While ultrasonography remains the gold standard for measuring tumor dimensions, EDI-OCT can be particularly helpful for evaluating small choroidal melanoma. Ultrasonography provides millimeter-level resolution, but for tumors less than 1 mm in thickness, EDI-OCT can provide
EDI-OCT has also been used in an attempt to distinguish between small choroidal melanoma and suspicious choroidal nevi. In one series of 37 eyes with small choroidal melanoma, choroidal shadowing and choriocapillaris thinning was found all cases.10 Compared with choroidal nevi of similar size, statistically significant EDI-OCT features of small choroidal melanoma included: intraretinal edema; loss of photoreceptors; loss of the external limiting membrane; loss of the inner segment-outer segment junction; irregularity of the inner plexiform layer; and irregularity of the ganglion cell layer. Elongated photoreceptors were observed overlying small choroidal melanoma in 49 percent of eyes, but this finding wasn’t observed in choroidal nevi.10
Swept-source OCT has recently been used to characterize choroidal lesions and may provide some advantages for pigmented tumors.11 SS-OCT uses a wavelength-tunable laser and a dual-balanced photodetector that provides superior imaging speed. Its adaptability to longer wavelengths allows for imaging of the choroid and greater penetration of melanin.12 In one series of 30 eyes with choroidal nevi, SS-OCT enabled visualization of intralesional details such as vessels, cavities
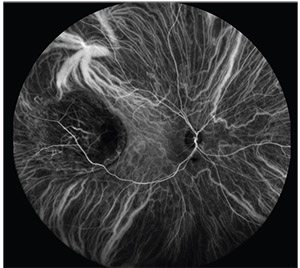 |
| Figure 4. Ultra-widefield indocyanine green angiography of choroidal melanoma demonstrating intrinsic vasculature. |
The newest form of OCT, optical coherence tomography angiography, is a non-invasive (i.e., dye-free) technique for imaging the retinal and choroidal vasculature. OCTA collects information regarding retinal and choroidal blood flow by comparing consecutive B-scans. OCTA may also help to distinguish choroidal melanoma from benign nevi by identifying unique vascular patterns. In a series of 11 patients (six with choroidal melanoma and five with choroidal nevus), OCTA demonstrated a
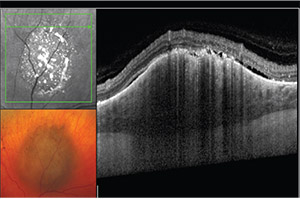 |
| Figure 5. EDI-OCT of a suspicious pigmented choroidal lesion with overlying orange pigment and subretinal fluid. The lesion demonstrated significant and quantifiable growth over the course of six months and was ultimately treated with I-125 plaque brachytherapy. |
OCTA can also be useful for both qualitatively and quantitatively assessing iatrogenic changes within the retina and choroid following treatment for choroidal melanoma. Images can be segmented to determine the degree of capillary dropout and nonperfusion in the superficial and deep layers of the retina, and in the choriocapillaris (Figure 6). A recent cross-sectional study compared OCTA findings in 10 eyes with choroidal melanoma imaged prior to therapy and 15 irradiated eyes with clinically apparent radiation retinopathy and/or optic neuropathy. In eyes with radiation-induced side effects, peripapillary retinal capillary density (PPCD) was lower in the treated eye and correlated with the radiation dose to the optic nerve as well as with the visual acuity outcome. In contrast, no significant difference was observed in PPCD in eyes with melanoma prior to irradiation compared with normal fellow eyes.15
Future Directions
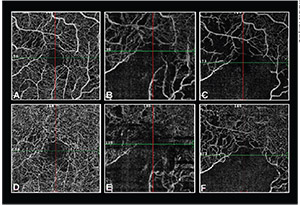 |
| Figure 6. OCTA showing a progressive decrease in macular capillary density 6 (A, D), 18 (B, E), and 24 (C, F) months following treatment with proton beam irradiation in the superficial retinal layers (top row) and deep retinal layers (bottom row). |
The widespread use of multimodal imaging for choroidal melanoma allows for superior characterization of tumors in a field that’s continually evolving. No one imaging technique alone serves as a gold standard for management. It’s the combination of imaging modalities that
Dr. Aronow is an assistant professor of ophthalmology at the Ocular Melanoma Center and Retina Service at Massachusetts Eye and Ear/Harvard Medical School. She can be reached at mary_aronow@meei.harvard.edu. She has no financial interest in any of the products discussed in the article.
1. Ciardella A, Brown D.
2. The Diabetic Retinopathy Study Research Group. Diabetic retinopathy study. Report Number 6: Design, methods, and baseline results. Report Number 7: A modification of the Airlie House classification of diabetic retinopathy. Invest Ophthalmol Vis Sci 1981;21:1-226.3. Mirchia K, Turell ME, Singh AD. Imaging modalities for uveal melanoma. European Ophthalmic Review 2012;6:1:56-63.
4. Schmitz Valckenberg S, Holz FG, Bird AC, et al. Fundus autofluorescence imaging: Review and perspective. Retina 2008;28:385-409.
5. Shields JA, Rodrigues MM, Sarin LK, et al. Lipofuscin pigment over benign and malignant choroidal tumors. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol 1976;81:5:871-81.
6. Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science 1991;254:1178-81.
7. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 2008;146:4:496-500.
8. Torres VL, Brugnoni N, Kaiser PK, et al. Optical coherence tomography enhanced depth imaging of choroidal tumors. Am J Ophthalmol 2011;151:586-93.
9. Shah SU, Kaliki S, Shields CL, et al. Enhanced depth imaging optical coherence tomography of choroidal nevus in 104 cases. Ophthalmology 2012;119:1066-72.
10. Shields CL, Kaliki S, Rojanaporn D, et al. Enhanced depth imaging optical coherence tomography of small choroidal melanoma: Comparison with
11. Francis JH, Pang CE, Abramson DH, et al. Swept-source optical coherence tomography features of choroidal nevi. Am J Ophthalmol 2015;159:1:169-76.
12. Mrejen S, Spaide RF. Optical coherence tomography: Imaging of the choroid and beyond. Surv Ophthalmol 2013;58:387-429.
13. Ung C, Laíns I, Silverman RF, et al. Evaluation of choroidal lesions with swept-source optical coherence tomography. Br J Ophthalmol 2018 Mar 31 [Epub ahead of print].
14. Ghassemi F, Mirshahi R, Fadakar K, Sabour S. Optical coherence tomography angiography in choroidal melanoma and nevus. Clin Ophthalmol 2018;12:207-14.
15. Skalet AH, Liu L, Binder C, et al. Quantitative OCT angiography evaluation of peripapillary retinal circulation after plaque brachytherapy. Ophthalmol Retina 2018;2:3:244-50.
16. Patel AV, Lane AM, Morrison MA, et al. Visual outcomes after proton beam irradiation for choroidal melanomas involving the fovea. Ophthalmology 2016;123:2:369-77.
17. Kines RC, Varsavsky I, Choudhary S. et al. An infrared
18. Kim IK, Lane AM, Jain P, et al. Ranibizumab for the prevention of radiation complications in patients treated with proton beam irradiation for choroidal melanoma. Trans Am Ophthalmol Soc 2016;114



