“OCT angiography is in its infancy, but we’re already finding that it gives us tremendous information about retinal vascular disease, such as branch vein occlusion and diabetic retinopathy, and it’s extremely helpful for the diagnosis of wet macular degeneration,” says David Boyer, MD, clinical professor of ophthalmology at the USC/Keck School of Medicine and partner at Retina Vitreous Associates Medical Group in Los Angeles.
Thomas Stone, MD, partner and chairman at Retina Associates of Kentucky, says that his practice is using it in three primary settings: retinal vascular disease; choroidal neovascularization; and select uveitis patients.
“In retinal vascular disease, like diabetes and vein occlusion, we use it if we are trying to determine macular perfusion in a patient,” Dr. Stone says. “To help us with visual prognosis, we’ll use OCT angiography in place of fluorescein angiography. With choroidal neovascularization, we have patients in whom we are not sure whether the change on their standard OCT or the change in their vision is a progression of dry AMD or a new development of an early choroidal neovascularization. This is true in both macular degeneration and ocular histoplasmosis. We feel that, with OCT angiography, we are able to identify whether there is some flow through these active vessels rather than just a scar.”
According to Dimitra Skondra, MD, PhD, assistant professor of ophthalmology at the University of Chicago and director of the J. Terry Ernest Ocular Imaging Center, “The amazing thing about OCT angiography is that it can reconstruct the information so that we can see blood vessels and blood flow in detail that we have never been able to see before without having to inject dye. We can get very valuable information about the vasculature. For the first time in imaging history, we can see all of the capillary plexi and the vasculature of the choriocapillaris in detail and in 3D
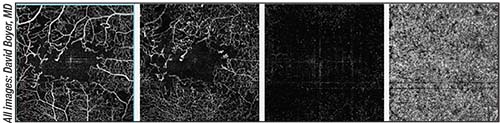 |
| Different depths of a retina with diabetic retinopathy imaged with OCT-A. Shown, from left to right, are the superficial, deep and outer retina, and the choriocapillaris. |
She adds that she uses OCT-A in almost all of her patients because it helps to provide more information for an accurate diagnosis, and it’s useful for showing how a patient is responding to treatment.
Retinal Vascular Disease
One of the primary uses of OCT-A is in patients with retinal vascular disease. “For diabetic retinopathy, we can easily visualize the parafoveal capillary bed, and we can see the degree of ischemia very easily without doing an invasive fluorescein angiogram,” Dr. Boyer says. “In fact, it gives us more information and is better than a fluorescein angiogram as far as determining whether there is ischemia in the superficial or deep capillary plexus. You can’t really image the deep capillary plexus with fluorescein angiography, which is a common site for ischemia.”
The same is true with branch vein occlusion. “With OCT angiography, we can see areas of ischemia, and we can determine its scope,” Dr. Boyer says.
A recent study has found that OCT angiography can quantify the retinal capillary microvasculature in patients with diabetes and can potentially be used to study the effect of systemic risk factors on the microvasculature.1 Previously, this was only possible using invasive techniques.
This prospective, observational study included 50 patients with type 2 diabetes with and without diabetic retinopathy. The researchers examined the retinal microvasculature with swept-source OCT angiography and semiautomated software to measure the capillary density index (CDI) and fractal dimension (FD) at the superficial vascular plexus (SVP) and deep retinal vascular plexus (DVP). Additionally, they collected data on histories of patients’ glycated hemoglobin A1c, hypertension, hyperlipidemia, smoking and renal impairment.
The mean glycated hemoglobin A1c of the 50 patients, whose mean age was 59.5 years, was 7.9 percent. The mean CDI at the SVP was 0.358 in patients with no diabetic retinopathy and 0.338 in patients with proliferative diabetic retinopathy. Additionally, the CDI at the DVP was 0.361 in patients with no diabetic retinopathy and 0.345 in patients with proliferative diabetic retinopathy. The mean FD at the SVP was 1.53 in patients with no diabetic retinopathy and 1.60 in patients with proliferative diabetic retinopathy, and the mean FD at the DVP was 1.55 in patients with no diabetic retinopathy and 1.61 in patients with proliferative diabetic retinopathy. The following systemic risk factors were associated with a lower CDI: hyperlipidemia (odds ratio: 9.82), smoking (odds ratio: 10.90), and renal impairment (odds ratio: 3.72). The following systemic risk factors were associated with increased FD: increased glycated hemoglobin A1c (≥ 8 percent) (odds ratio: 8.77) and renal impairment (odds ratio: 10.30).
Choroidal Neovascularization
OCT-A has also been used in patients with choroidal neovascularization. “In cases where we are concerned about whether there is a choroidal neovascular membrane present, such as in retinal pigment epithelial detachment, or in cases of type 3 choroidal neovascularization, such as a RAP lesion or polypoidal vasculopathy, with the en face imaging and the combination of the B-scans, we’re able to pick up early changes and follow or treat them. It also gives us an opportunity to see the response to treatment. Many times, we will treat a patient who has some leakage and perhaps some hemorrhage, and the OCT in general shows an area of leakage but the blood blocks everything on fluorescein angiography. When we treat them, they get better, and the blood goes away. Now, we can go back and look at that area very carefully and sometimes determine that there are no signs of choroidal neovascularization. In some cases, we are then able to stop treatment,” Dr. Boyer says.
According to Dr. Stone, in cases of choroidal neovascularization, surgeons must look for a flow pattern in the choroid. “Flow, rather than an inactive scar, indicates that treatment is required,” he says.
Dr. Skondra says that using OCT-A for wet AMD masqueraders has revolutionized her practice. “These are cases where the OCT shows something very similar to AMD, but you’re unsure if it’s an active membrane or not and if it should be treated with an intravitreal injection or not,” she says. “In these cases, a fluorescein angiogram may not always provide a straightforward answer because many conditions produce findings in the angiogram that look exactly like AMD. So, in these cases, there may be a very small membrane that an angiogram cannot pick up, or the patient may have a small neovascular membrane that you’re missing. Delaying treatment may cost the patient vision. In other cases, OCT and fluorescein angiogram findings, like pattern dystrophies and central serous chorioretinopathy, may mimic wet AMD. Additionally, there is no neovascular membrane, and no intravitreal injections are needed. Many patients who were treated for wet AMD come to me for a second opinion. With the combination of information from the fluorescein angiogram, the exam, the OCT and the OCT-A, I can tell with confidence whether a patient has wet AMD and treat him or her with injections when needed, or just observe the patient if it is an AMD masquerader, preventing unnecessary injections.”
A recent study has found that OCT-A can be used to perform qualitative and quantitative analyses of neovascular lesions.2 These researchers believe that, in the future, OCT angiography may provide biomarkers of activity and guide the evaluation, treatment and monitoring of neovascularization in AMD.
Macular OCT-A images were obtained, and morphologic
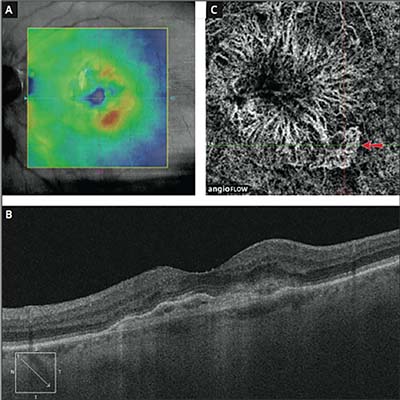 |
| In this patient, OCT showed minimal leakage and the OCT-A shows clearly defined CNV with new activity (arrow). |
The study included a series of 31 eyes: 11 eyes had active neovascular lesions at baseline and after consecutive follow-up after treatment with anti-VEGF therapy, and 20 eyes had quiescent neovascular lesions. Morphologically, all the quiescent neovascular lesions and 63.6 percent of the active NV lesions demonstrated a prominent central vessel, and active lesions demonstrated a greater rate of small vessels branching (82 percent) and peripheral arcades (82 percent) than quiescent lesions (30 percent and 40 percent, respectively). This was statistically significant. The lesion area and vessel density weren’t statistically significantly different after treatment or when compared to quiescent lesions, although quiescent lesions were reduced in area. Lesion pattern complexity was statistically significantly lower in the inner part of the lesion after treatment and in the total lesion of the quiescent neovascular lesion compared with the active neovascular lesions.
Uveitis
OCT-A can also be used in patients with certain types of uveitis, such as Behçet’s. In fact, a recent study found that OCT-A allows better visualization and characterization of perifoveal microvascular changes than fluorescein angiography in eyes with active Behçet uveitis.3 The deep capillary plexus seemed to be more severely involved than the superficial capillary plexus.
In this prospective, comparative, cross-sectional study, patients presenting with clinically active Behçet uveitis involving the posterior segment were evaluated using FA, spectral domain optical coherence tomography, and OCT-A.
The study included 44 eyes of 25 patients. Perifoveal microvascular changes were more frequently observed on OCT-A (95.5 percent) than on FA (59.1 percent). Disruption of the perifoveal capillary arcade, areas of retinal capillary nonperfusion/hypoperfusion, and perifoveal capillary abnormalities were observed more frequently using OCT-A than FA (40.9 percent vs. 25 percent, 86.4 percent vs. 34.1 percent, and 84.1 percent vs. 36.4 percent, respectively). Additionally, areas of retinal capillary nonperfusion/hypoperfusion were more frequently observed in the deep than in the superficial capillary plexus (81.8 percent vs. 63.6 percent).
The Future
“OCT angiography is in its infancy and has some inherent problems, such as shadowing and other artifacts giving us false images,” says Dr. Boyer. “Companies are working to overcome these types of problems. In the future, as it becomes better and better, we’ll be able to determine a greater degree of disease without doing any invasive treatment.”
Dr. Boyer notes that all of the machines are very good. “But, you can’t just do OCT angiography without having an en face image or B-scans looking for areas of geographic atrophy,” he says. “Many times, patients will appear as if they have an area of choroidal neovascularization, but what you are really seeing is an area of geographic atrophy, not choroidal neovascularization. Remember that OCT angiography doesn’t show leakage. Rather, it shows flow within vessels. In the beginning, practitioners may use OCT angiography as an adjunct as they become more familiar with the correlation between a fluorescein angiogram or indocyanine green and their findings on OCT-A. It has virtually eliminated the need for ICG angiography at this point. So, in some cases, it can visualize neovascularization without any signs of leakage before it can be seen on standard OCT.
So, in the future, it may be used as a screening test as part of our OCT to determine which patients need to be treated or followed more carefully.”
While there’s a role for OCT-A in the clinic, Dr. Stone believes this imaging technique needs to be able to image a broader area. “That’s currently being developed, and I expect continued improvements,” he says. “Another limitation is the degree to which OCT-A can show dynamic leakage. When I see leakage on my FA, that provides me with a hint of levels of activity. OCT-A just looks at structure. The question is, if diseases show leakage on fluorescein angiography, is there a way that can be shown on OCT-A?”
There are also practice management issues to consider. Dr. Stone explains that, in most geographic areas, payment is no different for OCT-A than it is for standard OCT. “You can’t charge for an angiogram; you have to charge for OCT, so you’re paying more to acquire an instrument that may not pay for itself if you don’t have a patient population that might benefit from the additional information,” he observes. “This being said, many of us use newer technology such as this because it provides information that is helpful for our patients, despite uncertain economics. Many smaller practices may need to figure out the financial side of it. If the economics could be worked out more thoroughly, this exciting technology could be available to more practitioners.” REVIEW
Dr. Boyer is a consultant to Optovue. Drs. Stone and Skondra have no financial interest in the products discussed.
1. Ting DSW, Tan GSW, Agrawal R, et al. Optical coherence tomographic angiography in type 2 diabetes and diabetic retinopathy. JAMA Ophthalmol 2017;135:4:306-312.
2. Al-Sheikh M, Iafe NA, Phasakkijwatana N, Sadda SR, Sarraf D. Biomarkers of neovascular activity in AMD using OCT angiography. Retina 2017 May 2. E-pub ahead of print.
3. Khairallah M, Abroug N, Khochtali S, et al. Optical coherence tomography angiography in patients with Behcet uveitis. Retina 2016 Dec 20. E-pub ahead of print.



