 |
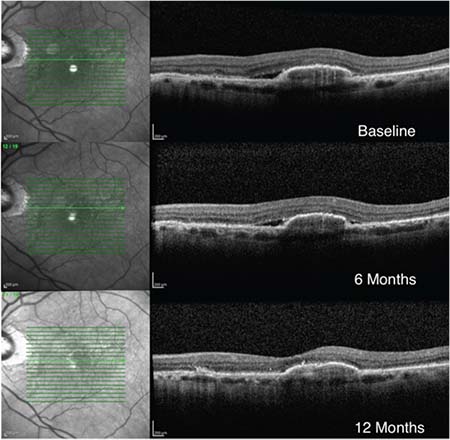
| Figure 1. An 83-year-old man who had suboptimal response to anti-VEGF monotherapy for six months (vision 20/25 at baseline and six months). After switching to a double-dose regimen the subretinal fluid resolved and vision improved to 20/20. |
The response to anti-VEGF therapy depends on a variety of factors, including the patient’s age, lesion characteristics, lesion duration, baseline best-corrected visual acuity and anatomic improvement on optical coherence tomography. We know from clinical trials that anti-VEGF therapies given as a standard regimen of monthly injections fail to control exudation in all patients. For example, the Comparison of Age-Related Macular Degeneration Treatment Trials research group reported that despite a monthly dosing regimen with anti-VEGF for a year, 53.2 percent of patients receiving ranibizumab 0.5 mg and 70.9 percent of patients receiving bevacizumab 1.25 mg had evidence of persistent subretinal fluid on OCT.3
Because of the lack of complete response to standard therapy in some patients with AMD, some clinicians and researchers have investigated the possibility of using higher-dose treatment. The LAST trial, the first prospective clinical trial of neovascular AMD to publish results of high-dose (2 mg) ranibizumab, reported that it has the potential to maintain or improve BCVA and anatomical outcomes in some patients with persistent or recurrent SRF or IRF secondary to neovascular AMD despite prior monthly intravitreal anti-VEGF therapy with the standard dose.4 Bascom Palmer retina specialist Phil Rosenfeld and his colleagues examined the tolerability of higher dose ranibizumab (up to 2 mg) and found that increasing the dose was well-tolerated, with a 40-percent rate of improvement in visual acuity that was similar to the standard regimen.5 Another study recently demonstrated
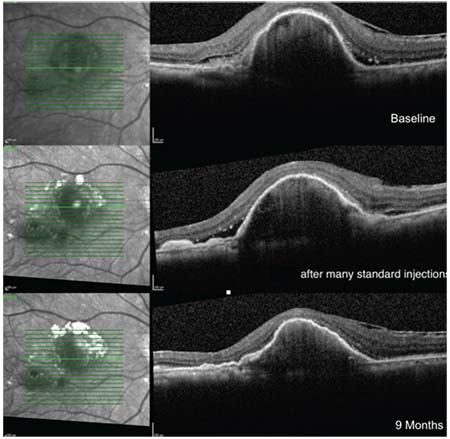 |
| Figure 2. A case of good response after switching to a double-dose regimen of anti-VEGF therapy. The images show resolution of subretinal fluid in a 73-year old woman with wet AMD and large pigment epithelial detachment despite many injections of anti-VEGF agents on a monthly regimen. Vision remained stable at 20/100 at all visits. |
In our clinical practice, we’ve used high-dose anti-VEGF agents with some success in a small cohort of cases of recalcitrant exudation in neovascular AMD (See Figures 1 and 2). This has provided us with an additional treatment option to consider in patients with an unsatisfactory response to standard therapy. A larger prospective, randomized clinical study will be necessary to validate this therapeutic approach and to identify features predicting a response to a high dose anti-VEGF therapy regimen. REVIEW
Dr. Sukpen is a fellow and Dr. Stewart a professor in the Department of Ophthalmology at the University of California, San Francisco Medical Center. Dr. Stewart can be reached at Jay.Stewart@ucsf.edu
1. Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA;2004;291:1900–1.
2. Lopez PF, Sippy BD, Lambert HM, et al. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci 1996;37:855–68.
3. CATT Research Group, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897–1908.
4. AT Fung, N Kumar, SK Vance, Slakter JS, Klancnik JM, Spaide RS, Freund KB. Pilot study to evaluate the role of high-dose ranibizumab 2.0 mg in the management of neovascular age-related macular degeneration in patients with persistent/recurrent macular fluid <30 days following treatment with intravitreal anti-vascular endothelial growth factor therapy (The LAST Study). Eye 2012;26:1181–1187.
5. Rosenfeld PJ, Heier JS, Hantsbarger G, Shams N. Tolerability and efficacy of multiple escalating doses of ranibizumab (Lucentis) for neovascular age-related macular degeneration. Ophthalmology 2006;113:4:623 e1.
6. Brown DM, Chen E, Mariani A, Major JC. Super-dose Anti-VEGF (SAVE) trial: 2.0 mg intravitreal ranibizumab for recalcitrant neovascular macular degeneration primary end point. Ophthalmology 2013;120:2:349-54.
7. Modjtahedi BS, Morse LS, Rashid S, Park SS. Double-dose ranibizumab for eyes with refractory exudative age-related macular degeneration. J Ocul Dis Ther 2013;1:18-23.