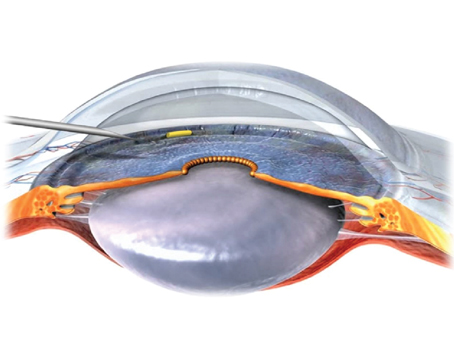
While the use of trabeculectomies has decreased in recent years—owing largely to new pharmaceutical alternatives and selective laser trabeculoplasty—tube shunt use has been relatively stable, even increasing a little in recent years. I believe that this is largely due to a growing sentiment among glaucoma specialists that glaucoma drainage devices can be used earlier in our armamentarium than they were previously.
One significant reason for this change is the data from the Tube vs. Trabeculectomy study, which has forced ophthalmologists to think about glaucoma drainage devices differently. For one thing, it clearly demonstrated that tube shunts are effective. The patient may need to use more medications, but shunts lower intraocular pressure and have fewer devastating complications such as hypotony and infection than trabeculectomies because of their lack of a traditional filtering bleb at the limbus. Also, the trial showed that in select cases drainage devices are a reasonable primary therapy, especially when the patient has certain refractory diagnoses.
Another factor in this gradual increase in shunt usage is that the move away from trabeculectomies by general ophthalmologists has generated an increased number of patient referrals to glaucoma specialists—and specialists tend to be more experienced with tube shunts, making them more likely to consider them as a treatment option. In previous years, a failed trabeculectomy would often lead to a second or third trabeculectomy; today, more glaucoma specialists in this situation are resorting to a tube shunt.
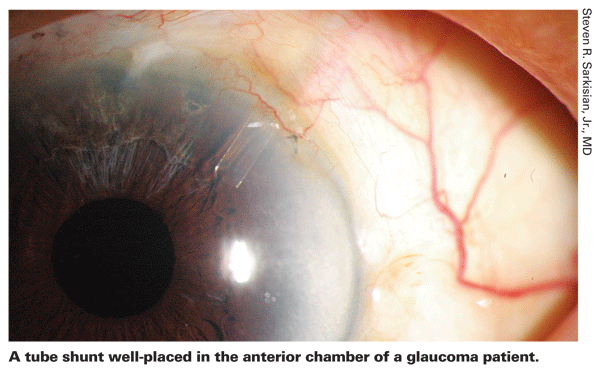
It's important to note that when I discuss glaucoma drainage devices, I'm primarily referring to the Ahmed, Baerveldt and Molteno devices, the first two being most widely used today. (A fourth option, the Krupin implant, is still available; however, it is less commonly used.) Although the Optonol ExPress shunt is currently my preferred glaucoma surgery option, I don't include it in this group. In my opinion, implanting the ExPress is small-incision glaucoma surgery, and more of a modification of trabeculectomy than a tube-shunt procedure.
Let's look at the most prevalent complications that occasionally arise following implantation of a tube shunt and how they can be managed effectively.
Hypotony and Shallow Chambers
Hypotony and shallow chambers have of-ten been associated with the use of glaucoma drainage devices. These are undesirable primarily because they can lead to choroidal effusions, choroidal hemorrhages or the drainage tube touching the cornea, all of which can result in vision loss.
Shallow chambers following glaucoma drainage device implantation are typically caused by over-filtration. One study that prospectively compared the silicone and polypropylene Ahmed valves reported the rate of hypotony to be 3 percent, with no statistically significant difference between the groups.1
Occasional cases of early hypotony may be unavoidable with the Ahmed because it's a valved implant with no suture placed around the tube to impede flow. However, I believe that most cases of hypotony seen with the Ahmed glaucoma valve result from mishandling of the device, leading to damage of the valve. The valve mechanism is quite sensitive; if you touch the box that it sits in with forceps while you're implanting it, you can damage it. Moreover, you're supposed to flush the tube of an Ahmed valve before implanting it. This means gently irrigating through the tube with a 30-ga. cannula to make sure there's some flow. If you push too hard or over-irrigate, you can damage the valve mechanism and end up causing hypotony.
In any case, if a patient does experience hypotony with an Ahmed valve, it will often be transient and resolve spontaneously. In my experience, if the eye has a pressure between 2 and 6 mmHg at postop day one or week one, that's rarely associated with a flat chamber or choroidal effusions; it's simply a low pressure. As the eye heals and scar tissue starts to form around the plate, causing some restriction of flow, the pressure will come up. I personally have not encountered persistent hypotony in the first month after implanting an Ahmed valve.
When implanting a non-valved Baerveldt shunt, the tube is restricted with a suture to prevent early hypotony. To avoid hypotony and shallow chambers, great care needs to be taken to make sure that your suture truly has caused a full restriction of the flow of fluid. Before you implant the device, try to irrigate the sutured tube with BSS through a cannula and make sure that no BSS comes out.
Unfortunately, despite your best efforts, early hypotony can still occur with a Baerveldt shunt because of inadequate closing of the tube with your suture—or because of aqueous leaking out around the tube through the hole in which it was placed. Because of the latter possibility, there's some controversy about the size of the hole you make for the tube; some have argued that using a needle larger than 23-ga. may cause this to happen. However, many others report no problems at all when using a 22-ga. needle. So the significance of the wound size remains unclear.
To some surgeons, the principal problem with the Baerveldt device is that the implant doesn't do much of anything until the suture is broken. Some surgeons make holes or fenestrations in the tube to control the pressure in the early postop period, but this is impossible to titrate and make reproducible. At the same time, you don't know exactly when the su-ture's going to break, and when it does break it's possible to have a sudden episode of hypotony.
Regardless of the type of implant you put in, some patients may have late hypotony because of ciliary body shutdown. The most effective response to this depends on the situation. You have to make sure that the system is intact—that you don't have erosion of the implant causing a leak. Erosion of the tube itself is rare and typically doesn't cause hypotony. Erosion of the plate is an even rarer complication, but it can be more serious and very difficult to repair. Often the whole implant has to be removed and a new implant inserted in a different quadrant.
To avoid the possibility of the tube touching the cornea, many surgeons are implanting the tube through the pars plana during vitrectomy, or, if the patient is pseudophakic, placing the tube in the sulcus to make sure it doesn't end up in contact with the cornea. Both methods are effective, but they obviously involve more work.
The Hypertensive Phase
Some surgeons have criticized the Ahmed valve for being associated with a so-called hypertensive phase, in which intraocular pressure rises for a period of time, accompanied by a high bleb over the plate caused by scar tissue. But at least one surgeon has hypothesized that the hypertensive phase may be a normal result of using this type of device—not a true complication—because it appears to happen in the majority of patients.2
If you encounter elevated IOP postop in a nonvalved implant such as the Baerveldt, and you're still in the early postop period and the suture has not broken, breaking the suture to restore flow is an obvious option. Some surgeons create another option by placing a "ripcord" suture through the tube and then stricturing the tube around that ripcord.
Then, if pressure is high several weeks after surgery, you have the option of pulling out the ripcord to increase flow. This approach involves more work, and it's surgeon-dependent, but it's a reasonable approach when working with a nonvalved implant.
It's also possible for obstruction of the tube to occur in the postoperative period, causing increased IOP. The diagnosis is usually straightforward, and treatment involves removal of the obstruction. Lasers can be used to re-move iris tissue, tissue plasminogen activator can be used for fibrin clots around the tube and vitreous can be removed with a laser or by vitrectomy.
The primary reason for elevated IOP postop is capsular fibrosis; when scar tissue initially forms, it can produce abrupt restriction in flow. In most cases, this accounts for the so-called hypertensive phase associated with either the Ahmed or the Baerveldt implant.
Typically, ocular massage—essentially, pushing on the eye—is used to address this. One study demonstrated that ocular massage forces aqueous through the tube and into the reservoir, blunting the effect of the hypertensive phase.3 Typ- ically, ocular massage, sometimes combined with aqueous suppression using either a beta blocker, carbonic anhydrase inhibitor or alpha-agonist, can get people through the hypertensive phase, and often the aqueous suppression can be stopped after several months. Digital massage and aqueous suppression allow the bleb to remodel and be lowered.
Many Baerveldt and Molteno users believe that reduced exposure to the inflammatory substance TGF-beta in the aqueous leads to reduction in scarring and smaller blebs, also helping to reduce the hypertensive phase; hence their preference for the Baerveldt or Molteno implants. However, this explanation is controversial and hasn't been studied extensively. One would expect an even higher rate of failure with valved implants if TGF-beta played a fundamental role in the success of glaucoma drainage implants. Also, the hypertensive phase occurs with both the Baerveldt and Molteno implants. We hope that future re-search will help to clarify whether or not this explanation regarding the role of TGF-beta is based on valid assumptions.
Ocular Motility
Problems with ocular motility such as strabismus and diplopia can occur following tube shunt implantation, though this is much more likely to happen in children than adults. In children, ocular motility disturbances have been found to occur in as many as one-third of cases. In contrast, in the Tube vs. Trabeculectomy trial, the tube group reported persistent diplopia in only five out of 107 adult patients.
In my experience, diplopia is more often associated with the Baerveldt shunt than the Ahmed, simply be-cause of the greater surface area of the shunt. However, I have seen diplopia with the Ahmed valve as well. Overall, the frequency of this type of problem is decreasing somewhat, thanks to the newest generation of implants, which are designed with fenestration holes to improve fixation by allowing fibrosis to occur through the holes. (In the rare cases in which I've had to remove a tube shunt, I've found that the holes did effectively serve this purpose, helping to anchor the shunt firmly to the sclera.)
Tube shunts are frequently used to treat aphakic glaucoma in children because surgeons have an aversion to performing trabeculectomies in children—the rate of long-term in-fection in a child following trabeculectomy with mitomycin-C is significant. Children tend to have different hygiene habits than adults and more active lives, and no one enjoys telling a child that she can't go swimming without risking infection. In congenital pediatric glaucoma the primary treatment is angle surgery, either trabeculotomy or goniotomy. However, once that has failed, the second procedure will often be a tube shunt.
In addition to motility disturbance, tubes in children have been associated with formation of cataract, tube-iris touch leading to pupil abnormalities, and tube-cornea touch. The last is a significant problem because a child's eye is often far more flexible than an adult eye, so that when you attempt to enter a child's eye with a needle to make the track for the tube, it's hard to position the needle track exactly where you want it. Furthermore, as the child's eye grows, the tube can pull out of the eye because more length is needed. In theory, this problem can be addressed by using special types of tubing or tube extenders,4 or by placing the tube in an S shape. The latter approach is intended to provide some built-in slack so that as the eye grows, the tube won't retract.
When an adult patient has a motility disturbance after a glaucoma drainage implant, I typically refer him to our strabismus specialist or to the orthoptist who works with our strabismus specialist. In many cases, the problem can be solved with a prism correction in the patient's glasses. If that's not possible, surgical intervention may be required, possibly including muscle surgery. Other options that have been attempted in this situation include removal of the fibrous capsule around the implant and implant plate size reduction. I've even seen reports of muscle surgery on the contralateral eye in patients with mild restrictions.
In general, the best preventive measure you can take is to be very careful during implantation, especially with the Baerveldt shunt, where motility problems seem to arise more often. Make sure that the wings of the implant are situated nicely under the muscles. It's been hypothesized that one wing being poorly placed, or placed above the muscle, may in-crease the likelihood of motility disturbance.
Tube shunts are sometimes im-planted in patients who've had corneal transplants; in these patients edema, corneal touching and graft failure have all been reported. These may also be concerns in patients who undergo Descemet's Stripping Endothelial Keratoplasty, although the data so far isn't sufficient to know for sure. When I refer a patient to a corneal colleague for one of these procedures, I give him license to trim any existing tube in order to prevent corneal touching, or to move the tube into the pars plana. If he's not comfortable doing that, I may participate in the surgery to manage that part of the procedure.
In any case, patients who have a corneal transplant and a glaucoma drainage device need to be told that the life of their corneal transplant will probably be shorter than it might be without the tube shunt. It's important to point out, however, that a corneal graft can be repeated while optic nerve damage caused by uncontrolled glaucoma cannot be undone.
Which Device Works Best?
In my experience, the Ahmed and Baerveldt implants work about equally well after several months, and retro- spective data has demonstrated that there's essentially no difference be-tween the two groups even at two years.5 However, this is a subject of some debate. And in fact, there is no concrete evidence so far that one is better than the other. We hope that the Ahmed vs. Baerveldt comparison trial will help to clarify the relative efficacy of these devices.
The main criticism leveled at the Baerveldt shunt is that implanting it requires a little more work because you have to occlude the tube with a vicryl suture, along with having to put the device under the muscles. As a result of the additional work, it takes a significantly longer time to place the larger-size Baerveldt than it does to place an Ahmed. However, if you place the 250-mm2 Baerveldt, you typically don't have to isolate the muscles as carefully; usually, only one wing is under the muscles or both are over them. (It's unclear whether there's a significant difference in IOP lowering with the 350- vs. 250-mm2 implants.)
There's also disagreement about which device is best to use in specific situations. In cases in which a low pressure is needed more quickly, such as in refractory, uveitic or neovascular glaucomas or corneal transplants, many surgeons would choose the Ahmed shunt. But if you ask 12 glaucoma specialists about their use of glaucoma drainage devices, you're likely to get 12 different answers. I think most people are currently choos- ing between these implants based solely on their personal experience, and possibly bias developed during their fellowship training.
A Valuable Option
Despite this lack of a clear-cut winner among the tube shunt alternatives, glaucoma drainage devices in general are a favored option for cases of refractory or pediatric glaucoma, and they are being used more often—and earlier—than they were in the past.
The idea of using a drainage device as primary surgery instead of a trabeculectomy is also gaining favor.
Soon, we should see some results from the Ahmed vs. Baerveldt study, and eventually we'll have data from upcoming studies investigating the use of tubes as primary surgery in virgin eyes. In the meantime, it's exciting to see how surgeons are being innovative in using these devices, looking for ways to help lower complication rates. The reality is that despite the occasional complications that can occur, glaucoma drainage devices are often the best option for a patient who needs to preserve vision by lowering pressure.
Dr. Sarkisian is clinical assistant professor of ophthalmology at the Dean A. McGee Eye Institute at the University of Oklahoma Health Sciences Center in
1. Ishida K, Netland PA, Costa VP, et al. Compari-son of polypropylene and silicone Ahmed Glau-coma Valves. Ophthalmology 2006;13:1320 – 1326.
2. Souza C, Tran DH, Loman J,
3. McIlraith I, Buys Y,
4. Sarkisian SR, Netland PA. Tube extender for revision of glaucoma drainage implants. J Glaucoma. 2007;16:7:637-9.
5. Tsai JC, Johnson CC, Kammer JA, Dietrich MS. The Ahmed shunt versus the Baerveldt shunt for refractory glaucoma II: Longer-term outcomes
from a single surgeon. Ophthalmology. 2006;113:6:913-7.