A look at the dropless/less-drops approaches employed by three surgeons follows, together with a discussion of the benefits, risks and regulatory environment surrounding the dropless and less-drops trends.
Why Go Dropless?
Post-cataract surgery drop regimens are notoriously labor intensive for doctors and staff as well as their patients, who tend to be older and more prone to physical and cognitive challenges that may make opening bottles, instilling drops and remembering dosing regimens a struggle. “It was confusing and detrimental to compliance when we had three separate bottles,” says Asim Piracha, MD, medical director of John Kenyon Eye Center in Louisville, Ky. “Patients had to make a bunch of check marks on sheets of paper, and they would still get confused.” Dr. Tyson adds that the drop burden on his patients created a disproportionate call volume for his staff. “Even with all the effort we’d put forth with counseling and schedules, our staff would continually get calls to answer patient questions about drops, not to mention refills. We’d be inundated: I’d say about 70 percent of our calls were related to postoperative drops,” he says.
Neal H. Shorstein, MD, of the Departments of Ophthalmology and Quality at Kaiser Permanente, Walnut Creek, Calif., believes topical ophthalmic therapy after cataract surgery might open avenues to the very infections it’s designed to prevent, via contaminated dropper bottles and touching of the eye. “Perhaps the less patients touch their eyes, the less chance there is of manipulating or burping fluid into the wound, allowing bacteria to enter, which may lead to endophthalmitis,” he says.
Practical difficulties and theoretical risks aside, the financial burdens topical eye drops pose to Medicare, Medicaid and patients are not insignificant. A 2015 report commissioned by Cataract Surgeons for Improved Eyecare and partially funded by Imprimis Pharmaceuticals, a maker of several dropless options,1 estimates that allowing patients to elect and pay for dropless cataract surgery (estimated to be $100 per prescription) would save Medicare and Medicaid approximately $7.1 billion between 2016 and 2025.
Applying Evidence to Practice
Injections at the time of cataract surgery have been used
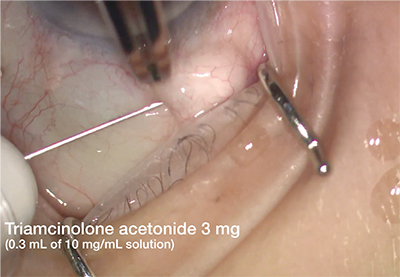 |
| Subconjunctival triamcinolone injection. This method of steroid injection is painless and does not induce postoperative floaters. Although the risk of patient response necessitating removal of the steroid is small, subconjunctival injection leaves the steroid accessible. |
When he noted an uptick in endophthalmitis cases in Kaiser Permanente’s Diablo service area in northern California, Dr. Shorstein looked to the ESCRS study. “In our department in 2007, we had a slightly higher incidence of endophthalmitis. We were doing about 3,000 surgeries a year at the time, and we noticed a few more infections than in previous years. That’s what prompted us to look in the literature and identify areas where we could address that,” he says.
Dr. Shorstein developed an antibiotic protocol dating from 2007 that was ultimately adopted throughout the Kaiser Permanente facilities in northern California. It includes injection of either cefuroxime or moxifloxacin after cataract surgery. “We inject 1 mg in 0.1 mL through the sideport incision at the end of the case. There was a recent report4 showing that using cefuroxime for stromal hydration keeps more drug around the wound site for a much longer period, up to 24 hours,” he says. “So a few of us inject a little bit of antibiotic into the cornea itself by stromal hydration, where it helps seal the wound, and apparently keeps the antibiotic at the wound site for many hours after surgery.”
For his drop-free approach, he follows the intracameral antibiotic injection with subconjunctival triamcinolone. “In about 2008, following some successful reports in the literature, a small group in my department started to inject subconjunctival triamcinolone and stopped using topical drops of any kind after cataract surgery. We subsequently studied outcomes and found that it worked very well,” he says. “We’ve been doing pretty much the same thing since then.”
Dr. Shorstein and colleagues evaluated the results of making intracameral antibiotic injections the standard of care in their service area over time. Their 2013 study5 showed that the incidence of endophthalmitis dropped from 3.13 per 1,000 during the initial 2007 and 2008 implementation period, to 0.14 per 1,000 in 2011, the last period reviewed. A 2016 comparative study6 of the records of 312,246 cataract procedures on 204,515 patients from 2005 to 2012 at Kaiser Permanente California showed that intracameral antibiotic injection (with an endophthalmitis incidence of 0.04 percent [0.4 per thousand]), was superior to topical antibiotic alone, (with an endophthalmitis incidence of 0.07 percent [0.7 per 1,000]), and that antibiotic drops didn’t confer any additional benefit when instilled after intracameral injections.
Louisville’s Dr. Piracha has been using a mixed dropless and less-drops protocol for more than three years. The antibiotic injection regimen is derived from the 2007 ESCRS study protocol. “If our patients don’t have a cephalosporin allergy, we’ll use cefuroxime intracameral injections on everybody at the conclusion of the case. We also do sub-Tenon’s triamcinolone,” he says. Dr. Piracha’s patients also get a postoperative combo drop consisting of moxifloxacin, prednisolone acetate and bromfenac for added coverage. “Some people still get the three separate prescription bottles,” he says, “but most will go with the bottle that’s compounded, which is around $50, cash only, for all three medicines. We can usually use the same bottle for both eyes, so most of our patients end up paying just $25 per eye. It’s easy because they use just one bottle four times a day for four days, tapering down over a four-week period.”
Dr.
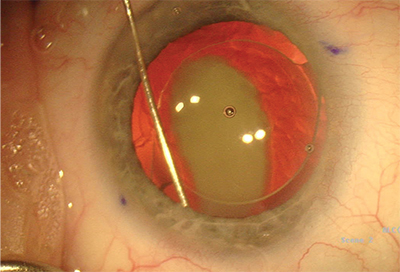 |
| Transzonular intravitreal injection with Tri-Moxi-Vanc. The triamcinolone plume is clearly visible, and patients may notice floaters for about a week. Although there is a small risk of IOP spike, the steroid remains active in the eye for several weeks postop to fight edema. |
The transzonular intravitreal approach does carry a risk of zonular damage and consequent capsular bag instability, which in cataract surgery can cause decentration of the IOL; this must be weighed against the remote but serious risks of retinal detachment and hemorrhage with pars plana intravitreal injection.7
Dr. Tyson differs from Dr. Shorstein and Dr. Piracha in electing to do an intravitreal injection, intended to maximize the activity of the antibiotic. “The ESCRS study was a milestone, showing that there was a fivefold reduction in infectious endophthalmitis by putting intracameral cefuroxime, a second-generation cephalosporin, into the anterior chamber. Putting it into the anterior chamber works,” he says. “Then the Shorstein study in 2013 showed a 22-fold reduction in endophthalmitis, and it really didn’t matter which antibiotic you used when you placed it intracamerally. The problem with an intracameral approach is that there’s a turnover of fluid in the anterior chamber every two to four hours. The vitreous has a much slower turnover and acts as a depot. This has not been studied clinically and there is no peer-reviewed data on it, but theoretically, there’s a 12-hour turnover when you put antibiotic into the area where you’re most concerned about bugs: the vitreous. You lay that stuff in there, and it gets slowly resorbed and released over at least a 12-hour period.”
Study Your Own Patients
In addition to inferring his ideal injection approach, Dr. Tyson studied his clinical outcomes to help ensure safety and improve results with Tri-Moxi-Vanc. Of a retrospective review of 1,541 procedures8 at his facility using injected Tri-Moxi-Vanc, he says, “We had very few pressure spikes: We saw less than one percent, and all of those patients were easily handled with medication, although many did not even require it. It was a transient phenomenon.”
Dr. Tyson’s study reports that breakthrough inflammation at postop days 14 through 21 was 9.2 percent, but that the proportion of those instances of inflammation was smaller during the second half of the study period. “As time has gone on, I’ve recognized that patients who have darker irises tend to have more of a rebound iritis phenomenon, so we give just a little bit more Tri-Moxi-Vanc. That seems to have reduced the rebound iritis by almost 50 percent in those patients,” he reports. “We now realize that there are things you can do to reduce the incidence of even the few issues that might happen.” Although the visually significant CME rate at days 14 through 21 was 2 percent, he noted that 35 percent of the patients who developed CME had had a previous episode of iritis, and so he began to start his postop iritis patients on a preventative nonsteroidal. “That’s the beauty of looking at your data,” he says. “We know who’s going to possibly have issues, so we try to be a little preemptive. You can use either a less-drops preparation, or you can use just use a nonsteroidal.” Another group at risk of CME consisted of patients with epiretinal membrane, so they, too, were started on a postop nonsteroidal in addition to the injection.
All three surgeons have made dropless and less-drops procedures work for them by staying alert to patterns in the literature and their own clinical experience, and then adjusting their protocols accordingly. Dr. Piracha says, “It’s come down to four or five iterations in the last couple of years, trying to find the best and the safest.” Although his practice tried intravitreal Tri-Moxi, a proprietary mix of triamcinolone and moxifloxacin from Imprimis, they saw inflammatory reactions within their first 1,000 cases that prompted them to switch to separate cefuroxime and triamcinolone injections.
With intravitreal steroid, floaters do occur, but Dr. Tyson says that they are short-lived and haven’t diminished the “wow factor” of cataract surgery for his patients. “Patients experience a few floaters here and there, but we warn them ahead of time so they’re not freaked out by them, and they’re gone in about five to seven days.” He adds that using an inferonasal approach with the TMV helps prevent patients from perceiving any floaters in their central vision.
Assessing the Risks
When mixing agents and injecting them into patients’ eyes, the potential for damage from human error is always present. One adverse event reported in the literature was an outbreak of toxic anterior segment syndrome among 12 cataract patients who were inadvertently injected with Moxeza (Alcon), a brand of moxifloxacin containing preservatives, instead of Vigamox.9 Compounding your own agents entails the risk of contamination or dilution mistakes. “Dilutional error from compounding drugs has been reported in the literature that has caused macular edema and toxic anterior segment syndrome,” notes Dr. Shorstein.
Dr. Piracha relies on an FDA-compliant outsourcing facility that formulates medications per patient-specific prescriptions. “We don’t even want to come close to messing with the rules,” he says. “Each patient has his or her own single-use vial of antibiotic made up in sterile packaging. The compounding pharmacy makes our cefuroxime; they also make preservative-free triamcinolone for each patient, individually wrapped for a single use.”
“We feel that since there is no FDA-approved manufactured drug that is available—which would certainly be our first choice—sourcing antibiotic for intracameral injection from an FDA-registered compounding facility is a good second choice,” adds Dr. Shorstein. Like Dr. Piracha’s practice, Dr. Shorstein’s department gets its postoperative medications directly from a compounding pharmacy that is registered with the FDA pursuant to Section 503(b) of the Drug Quality & Security Act of 2013, which among other things, subjects compounders to FDA inspections. The Imprimis injectables that Dr. Tyson uses also come from an FDA-registered facility. Referencing the 2012 meningitis outbreak traced to steroids mixed at the New England Compounding Center in Massachusetts that killed 64 and sickened over 700 others, Dr. Tyson says of the response to the resulting enhanced legislation, “It’s really very remarkable how some of these compounding facilities have come to the forefront in addressing safety to make us feel a whole lot more secure about using them.”
The risk of IOP spike when injecting a steroid initially prompted Dr. Shorstein to go beyond simple informed consent. “Early on, we advised our patients that there were no FDA-approved drugs for endophthalmitis or CME prophylaxis, and that the steroid injection could increase their intraocular pressure, and if that occurred, we would either need to add drops for a short time or excise the depot of triamcinolone,” he recalls. “Then my research team published a study recently.10 We looked at over 16,000 eyes in my department and looked at three groups: One group got topical steroid after surgery; one group got topical steroid and nonsteroidal anti-inflammatory drops in addition to the steroid; and the third group were the patients who just got the injection of triamcinolone. We found there was no difference in CME in the groups that had topical steroids or the group that got the injection. There was no difference in postoperative iritis diagnoses,” he says. The study also showed that the percentages of eyes with intraocular pressure spikes above 30 mmHg were about the same in patients who got subconjunctival injections as in those who got topical prednisolone drops. “So I really stopped the additional counseling to individual patients after that study, other than including in their preoperative educational materials that they would be receiving injection and there was a small risk of increased intraocular pressure,” Dr. Shorstein says.
Regarding Vancomycin
Dr. Shorstein’s department will rarely use vancomycin, generally only in patients with known penicillin, cephalosporin or fluoroquniolone allergies. He acknowledges that for certain immunosuppressed patients, or those who are already colonized with MRSA, vancomycin injection may be beneficial. Dr. Piracha doesn’t use vancomycin at all. Recent studies linking vancomycin to postoperative hemorrhagic occlusive retinal vasculitis (HORV),11 a rare condition characterized by initial painless blurred vision, retinal vascular occlusion and hemorrhage leading to profound vision loss, have given doctors pause. “In general, we respect the recent reports that have shown the devastating loss of vision in patients who are injected with vancomycin, and at Kaiser Permanente Northern California, we aren’t routinely injecting it,” says Dr. Shorstein.
Dr. Tyson initially responded to reports associating vancomycin with HORV by eliminating it from his injections. “I switched to Tri-Moxi for a little bit, then switched back to TMV,” he says. “HORV scares you, but it’s just not worth it to me, for something that seems idiosyncratic from a statistical standpoint, to potentially do the patient a disservice by possibly not treating those gram-positive bugs.” He also notes that no cases of HORV have been reported using the product he injects at the conclusion of cataract surgery.
Vancomycin has long been considered an antibiotic of last resort, and its use hasn’t been encouraged for cataract surgery.12 Dr. Tyson doesn’t believe that injecting vancomycin into the eye is a contributor to vancomycin resistance, however. “Either intravenously or as a drop, we might only partially kill bugs and then those bugs could regenerate,” he says, “but inside the eye we have a closed system. It’s highly unlikely that we’d create a resistant bug by putting it inside the eye.”
Regulatory Dilemmas
The use of intracameral prophylaxis after cataract surgery is ubiquitous in Europe, and even jointly supported by governmental recommendations and national ophthalmological societies in France and Denmark.13 Although American surgeons may believe that dropless approaches are safe and effective, they may also be deterred by the lack of an FDA-approved agent, as well as Medicare and Medicaid regulations that compel them to absorb the cost of the injections.
To date, the FDA has received no applications for an antibiotic endophthalmitis preventative for injection. Given that the incidence of endophthalmitis after cataract surgery is estimated to be approximately 2,000 eyes among some 3 million cataract surgeries annually in the United States,13 the costs of recruiting patients for a powerful randomized controlled trial would be astronomical; given the extant evidence that injections are superior to topical antibiotics, surgeons might have ethical qualms about enrolling patients even if such a study were economically feasible.13
Dr. Shorstein is among a group of thought leaders seeking a path to FDA approval. “I had the good fortune to meet twice with Wiley Chambers, MD, who is a deputy director for ophthalmology products in FDA, along with David Chang, MD, the late Peter Barry and Nick Mamalis, MD, on behalf of ASCRS,” he says. “Dr. Chambers felt that a prospective antibiotic study was feasible, and other avenues were probably less likely to be successful. I know that ASCRS has been thinking for quite a while now about how to achieve that holy grail of an FDA-approved drug. I don’t think there’s a clear solution yet, but we’re working on it.”
Dr. Tyson is less optimistic about FDA approval, but would like to see CMS change its posture on intracameral injections. CMS currently considers such injections as part and parcel of cataract surgery, not billable to the patient or recoverable to the surgeon. “We’re trying to get this covered by Medicare. We’re absorbing the costs ourselves as surgeons because it’s entirely bundled within the surgery reimbursement. It would be great if we could get a pass-through designation for it, or if we could have patients sign an ABN stating that they could pay for it: We’re saving patients hundreds of dollars and we’re saving the Medicare system about 7 billion dollars projected over 10 years just by using this type of formulation versus drops,” he says, referring to the CSIE study.
The regulatory future of dropless and less-drops cataract surgery in the United States remains uncertain, but the protocol is becoming increasingly available with each passing year. Surgeons who want to implement it must look at the existing evidence, choose their therapeutic agents carefully and be willing to study their own outcomes to offer the most convenient and cost-effective treatments to patients without compromising safety and efficacy. “We’re always looking for the best scenario for our patients,” says Dr. Piracha. “It’s evolved multiple times over the last four or five years, and it will probably continue to evolve.” REVIEW
Dr. Tyson is a consultant for Imprimis Pharmaceuticals, Alcon, Allergan and Ocular Therapeutix. Dr. Piracha has received speaker’s fees from Abbott Medical Optics. Dr. Shorstein reports no financial interests.
1. Andrew Chang and Co., LLC. “Analysis of the economic ipacts of dropless cataract therapy on Medicare, Medicaid, state governments, and patient costs.” October 2015.
2. Christy NE, Lall P. A randomized, controlled comparison of anterior and posterior periocular injection of antibiotic in the prevention of postoperative endophthalmitis. Ophthalmic Surg 1986;17(11):715-71.
3. ESCRS Endophthalmitis Study Group. Prophylaxis of po stoperative edophthalmitis following cataract surgery: Results of the ESCRS multicenter study and identification of risk factors. Journal Cataract & Refract Surg 2007;33(6):978-88.
4. Moosajee M, Tracey-White D, Harbottle RP, Ferguson V. Safety profile of stromal hydration of clear corneal incisions with cefuroxime in the mouse model. J Ocul Pharmacol Ther 2016;32(7):469-75.
5. Shorstein NH, Winthrop KL, Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. J Cataract Refract Surg 2013;39(1):8-14.
6. Herrinton LJ, Shorstein NH, Paschal JF, et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology 2016;123(2):287-94.
7. Kumar BV, Harun S, Prasad S. Letter to the Editor. Trans-zonolar delivery of intravitreal triamcinolone acetonide in the management of pre-existing macular oedema during cataract surgery. Acta Ophthalmologica 2006;84(3):438-9.
8. Tyson SL, Bailey R, Roman JS et al. Clinical outcomes after injection of a compounded pharmaceutical for prophylaxis after cataract surgery: A large-scale review. Curr Opin Ophthalmol 2017;28(1):73-80.
9. Braga-Mele R, Chang DF, Henderson BA, Mamalis N, et al. Intracameral antibiotics: safety efficacy, and preparation. J Cataract Refract Surg 2014;40:2134-2142.
10. Shorstein NH, Liu L, Waxman MD, Herrinton LJ. Comparative effectivenesss of three prophylactic strategies to prevent clinical macular edema after phacoemulsification surgery. Ophthalmology 2015;122(12):2450-6.
11. Witkin AJ, Shah AR, Engstrom RE et al. Postoperative hemorrhagic occlusive retinal vasculitis: Expanding the clinical spectrum and possible association with vancomycin. Ophthalmology 2015;122(7):1438-51.
12. No authors listed. Recommendations for preventing the spread of vancomycin resistance. Hospital Infection Advisory Control Committee (HIPCAC). Infect Control Hosp Epidemiol 1995;16(2):105-13.
13. Javitt JC. Intracameral Antibiotics reduce the risk of endophthalmitis after cataract surgery: Does the preponderance of the evidence mandate a global change in practice? Ophthalmology 2016;123:2:226-3.



