Workup, Diagnosis and Treatment
Magnetic resonance imaging of the brain and orbits with and without contrast was obtained emergently and demonstrated “diffusely enlarged and edematous extraocular muscles with sparing of the tendinous insertions” (Figure 2). Additional laboratory and imaging studies were obtained, including a complete blood count with differential, erythrocyte sedimentation rate, c-reactive protein test, Lyme antibodies, thyroid testing, serum angiotensin converting enzyme, blood urea nitrogen test, creatinine, IgG4 antibodies, antineutrophil cytoplasmic antibodies, antinuclear antibodies, lactate dehydrogenase, uric acid, Quantiferon-gold, and a chest X-ray. While in the emergency room, the patient received empiric treatment with 6 mg of IV dexamethasone but didn’t have any significant improvement in his symptoms or exam. In addition, he had a thyroid-stimulating hormone elevated to 16.11 (normal 0.3-5.0 mU/mL) but a normal level of free T4. ESR was also elevated, but the CBC, CRP and chest X-ray were normal. All other testing was pending. Given the absence of optic nerve involvement, he was started on 40 mg of prednisone daily and discharged with a plan to follow up in the Wills Eye oculoplastics service.
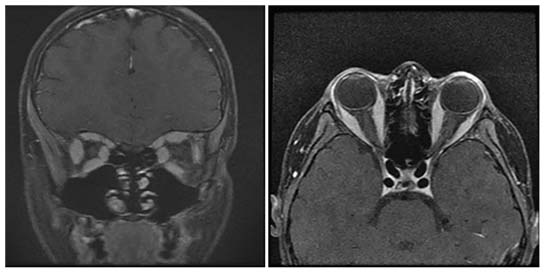 |
| Figure 2. T1-weighted, post-contrast MRI of the orbits showing diffuse extraocular muscle enlargement without tendon sparing. |
At follow-up three days later, the patient reported resolution of the double vision. Ocular examination revealed decreasing proptosis of the right globe and significantly improved extraocular motility; the motility deficit was more paretic than restrictive. A secondary review of the MRI called into question the presence of tendon sparing. All of the remaining testing was normal, including a thyroid-stimulating immunoglobulin. This compilation of findings and the rapid response to steroids made idiopathic orbital inflammation the most likely diagnosis.
A repeat TSH measured two weeks later was 8.750; free T4 and T3 were normal. The patient was referred to a pediatric endocrinologist who didn’t find any evidence of thyroid disease and explained that elevations in TSH can occur in peripubescent children. The patient’s extraocular motility deficits, ptosis and proptosis completely resolved two weeks after starting steroids, which were slowly tapered (Figure 3). At his follow-up appointment three months after initial presentation, he was off all steroids without any signs or symptoms of recurrence.
Discussion
The differential diagnosis of extraocular muscle enlargement in the pediatric population is broad and includes inflammatory (thyroid eye disease, idiopathic orbital inflammation), infectious and neoplastic etiologies.
The initial findings in our case suggested thyroid eye disease as an etiology. Pediatric thyroid disease is a rare condition with an incidence of 0.1 to three cases per 100,000 children, and thyroid-associated ophthalmopathy (TAO) has an incidence of 0.8 to 6.5 cases per 100,000 children with thyroid disease.1,2,3 The mean age at diagnosis is 12.4 to 15 years, with two-thirds being postpubescent adolescents.1,3 TAO is generally milder in children, and the most common manifestations are lid retraction, proptosis and soft tissue involvement.1,2,3 Restrictive strabismus and optic neuropathy are uncommon in the pediatric population, but when they do occur, are more likely complications in postpubescent children.1,2 This may be related to an increase in smoking prevalence in older children, which is a known risk factor for TAO.1 Although the patient in our case had an extensive family history of thyroid disease and an elevated TSH, the patient’s age and paretic motility deficits were inconsistent with TAO, and the TSH was explained by peripubescent hormonal changes.
As the clinical course unfolded, idiopathic orbital inflammation (IOI) became the most likely diagnosis in the presence of an unrevealing workup and rapid response to steroids. IOI was first described in 1905 and was initially referred to as “orbital pseudotumor” because of its appearance as a benign, non-infectious, space-occupying lesion in the orbit, or enlargement of an orbital structure.4 A diagnosis of exclusion, its etiology is still unknown, although autoimmune disorders, upper respiratory infections, viral illnesses and aberrant wound healing have all been proposed as possible mechanisms for pathogenesis.4 IOI can be divided into two anatomic subgroups: nonmyositic (e.g., dacryoadenitis, orbital fat inflammation) and myositic.
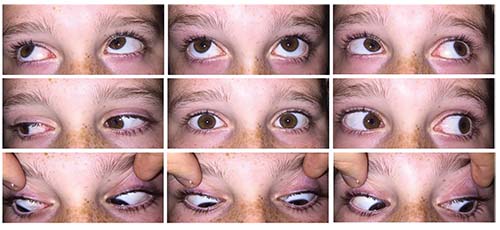 |
| Figure 3. Extraocular motility with dramatic improvement shortly after starting oral steroids. |
A consensus study based on surveys completed by members of the Orbital Society defined a set of criteria for the diagnosis of IOI.5 Orbital pain, and pain with eye movements in the case of myositic IOI, are important criteria, as well as an acute or subacute onset (days or weeks), usually with a slow progression. Since IOI is diagnosed in the absence of an underlying etiology, a history of orbital-related systemic disease shouldn’t be present, nor should thyroid disease in the setting of myositic IOI. While CT or MRI are important for localizing the anatomic compartment involved, serologies such as inflammatory markers and autoantibodies play a limited role as they may be falsely negative in patients with disease limited to the orbit or with mild systemic disease. Nonmyositic IOI requires a pathologic diagnosis, with histopathology usually showing a nonspecific, polymorphous infiltrate of small, well-differentiated lymphocytes (primarily T cells), plasma cells, and neutrophil and eosinophil granulocytes.6 Myositic IOI, on the other hand, can be diagnosed by an avid response to corticosteroids within 48 hours of initial administration.
A review of 30 cases of pediatric IOI found that the mean age of onset was 11 years, with a female predominance. Headache was the most common constitutional symptom, and signs on exam were similar to adults, including periorbital edema, ptosis and proptosis. In contrast to pediatric TAO, more than one-third of children with IOI had decreased extraocular movements. The most common radiographic findings included dacryoadenitis, orbital mass and myositis.7 Another case series of 29 pediatric patients with IOI found that only 10.3 percent had bilateral involvement at the onset of disease, but 44.8 percent eventually developed bilateral disease. Patients with bilateral disease were more likely to have a motility disturbance (76.9 percent) compared to those with unilateral disease (56.3 percent). In addition, 92.3 percent of patients with bilateral disease developed recurrences, with an average of 4.7 episodes.8 Females were also more likely to develop recurrent disease.7
Treatment of pediatric IOI, like the adult form of the disease, is dependent on disease severity, clinical and radiologic findings and initial versus recurrent disease. One case series reported that 80 percent of patients were treated with oral steroids with a starting dose of 1mg/kg/day.7 Appropriately responding cases showed improvement in two to three days.7 For those cases that did not respond, patients received IV steroids, rituximab, IVIG and/or radiation, with 83 percent achieving complete resolution at last follow-up. Local treatment in the form of a perimuscular injection of betamethasone suspension in 12 adults with orbital myositis led to complete resolution within one to two weeks and only one recurrence 14 months after the initial injection.9
Orbital myositis may not need to be routinely biopsied.5,7 Indications for biopsy include the absence of pain, subacute onset, a restrictive pattern of extraocular motility deficits, and either a failure to respond to corticosteroids or a disease recurrence while tapering. Furthermore, atypical imaging findings that may be indications for biopsy include irregular edges, nodularity, focal intramuscular mass on imaging, orbital fat infiltration, lacrimal gland enlargement or swelling, orbital nerve enlargement, and/or sinus disease.5
In conclusion, IOI is an orbital inflammatory disease of unknown etiology that occurs in the absence of known systemic disease with orbital manifestations. Although often unilateral, bilateral disease is more common in the pediatric population, as demonstrated in our case. IOI usually responds to corticosteroids but may require biopsy followed by other types of immunosuppression in refractory or recurrent cases. REVIEW
1. Gogakos AI, Boboridis K, Krassas GE. Pediatric aspects in Graves’ orbitopathy 2010;7:234-244.
2. Holt H, Hunter DG, Smith J, et al. Pediatric Graves’ ophthalmopathy: The pre- and postpubertal experience. J AAPOS 2008;12:4: 357-360.
3. Durairaj VD, Bartley GB, Garrity JA. Clinical features and treatment of Graves ophthalmopathy in pediatric patients. Ophthal Plast Reconstr Surg 2006;22:7-12.
4. Birch-Hirschfeld A. Zur diagnostic and pathologic der orbital tumoren. Ber Dtsch Opthalmol Ges 1905;32:127–35.
5. Mombaerts I., Bilyk JR, Rose GE, et al. Consensus on diagnostic criteria of idiopathic orbital inflammation using a modified delphi approach. JAMA Ophthalmol 2017;135:769-776.
6. Mombaerts I, Goldschmeding R, Schlingemann RO, et al. What is orbital pseudotumor? Surv Ophthalmol 1996;41:66-78.
7. Spindle J, Tang SX, Davies B, et al. Pediatric idiopathic orbital inflammation: Clinical features of 30 Cases. Ophthal Plast Reconstr Surg 2016;32:270-4.
8. Mottow LS, Jakobiec FA. Idiopathic inflammatory orbital pseudotumor in childhood. I. Clinical characteristics. Arch Ophthalmol 1978;96:1410-7.
9. El Nasser A Mohammad A. Local steroid injection for management of different types of acute idiopathic orbital inflammation: An 8-year study. Ophthal Plast Reconstr Surg 2013;29:286-9.



