Three randomized, double-masked, placebo-controlled, conjunctival antigen-challenge-model clinical trials established the efficacy of Zerviate. The trials studied patients with a history of allergic conjunctivitis. The most commonly reported adverse reactions (ocular hyperemia, instillation site pain and reduction in visual acuity) only occurred in 1 to 7 percent of patients treated. The recommended dose of Zerviate is one drop in each affected eye, twice daily (about eight hours apart).
For more information on Zerviate 0.24%, visit nicox.com.
Eyefficient’s Portable Fundus Camera
In mid-July, Eyefficcient, through a partnership with MediWorks, introduced its new handheld fundus camera, the FC160, in the U.S. This
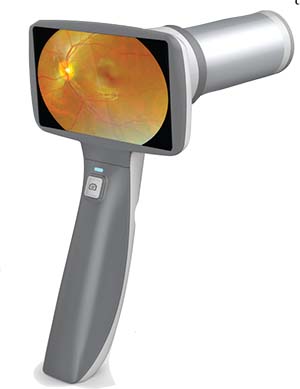 |
The camera features a rechargeable lithium-ion battery. Eyefficient also says that the portable fundus camera weighs only 420 g. Eyefficient highlights the convenience of its MediWorks products, claiming that with the Portable Fundus Camera and its other products, doctors are able to capture retinal images with ease, using features such as the fundus camera’s 3.97-inch touch screen and five internal fixation targets.
For more information on Eyefficient’s Portable Fundus Camera, visit eyefficient.com.
Bausch + Lomb’s Fortifeye Capsular Tension Ring
Bausch + Lomb has announced the launch of its Fortifeye capsular tension ring. The ring is a pre-loaded, sterile, non-optical implant for the stabilization of the crystalline lens capsule due to weak or partially absent zonules in adult patients who are undergoing cataract extraction.
Some indications for its use include primary zonular weakness (Marfan syndrome), secondary zonular weakness (trauma or vitrectomy), cases of zonulysis, cases of pseudoexfoliation and cases of Weill-Marchesani syndrome.
The implants are made of one continuous piece of polymethyl methacrylate. They are available in both counterclockwise and clockwise insertion options, 10-mm, 11-mm and 12-mm diameters.
For more information on the Fortifeye capsular tension ring, visit bausch.com.
FDA Clearance for Mynosys’ Zepto
The FDA recently approved the Zepto capsulotomy system, a disposable handpiece that uses a combination of suction and low-energy pulses for high-quality, fast capsulotomies.
The device can be integrated into standard cataract surgery and is inserted through a 2.2-mm incision. The FDA clearance comes following the results of studies published in February 2016, one of which found that the Zepto produced complete, round capsulotomies with minimal zonular stress in live rabbits and human cadaver eyes.
Mynosys highlights the Zepto’s unique design, which incorporates nanopulse technology that reduces surgical time. It has a four-millisecond cutting time and Mynosys says it creates a consistent and uniform capsulotomy.
For more information on Mynosys’ Zepto, visit mynosys.com.



