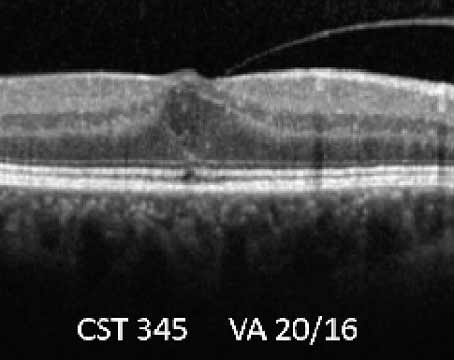
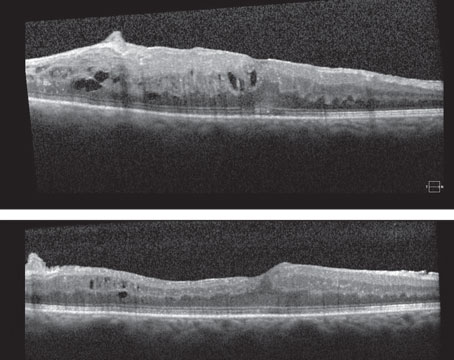
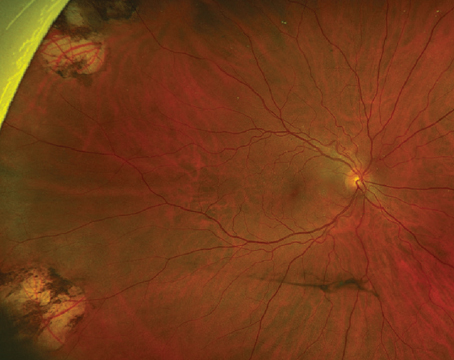
Treatment of neovascular age-related macular degeneration has been revolutionized over the past few years by the advent of agents that inhibit vascular endothelial growth factor. The emergence of these agents is a direct result of research into the cellular and molecular mechanisms mediating physiological and pathological angiogenesis, including choroidal neovascularization, the hallmark of neovascular AMD.
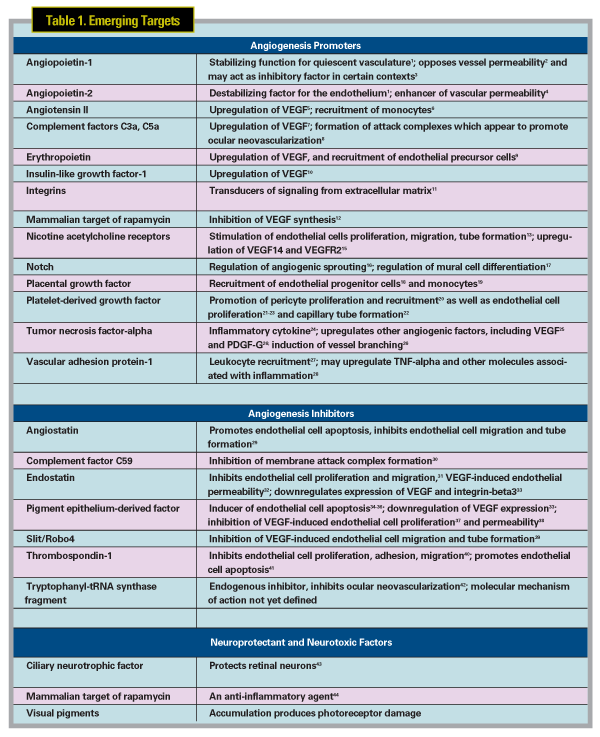
While the success of the anti-VEGF therapies has been rewarding, research into the pathogenesis and treatment of AMD continues. Preclinical studies designed to elucidate the molecular pathways involved in AMD pathogenesis have identified a variety of potential targets, most of them linked directly or indirectly to inflammatory processes (See Table1),1-44 with some candidate agents now in early-phase clinical trials. Many of these studies are based on blocking the effects of promoters of angiogenesis, while others are exploring the role of inhibitors of angiogenesis in the formation of CNV, and still others are directed to the development of treatments for geographic atrophy, a potential precursor to neovascular AMD and a major cause of vision loss in its own right. This review will discuss the most promising of these targets, ordered according to current clinical phase of development. Finally, there is a brief discussion of potential targets that are undergoing preclinical evaluation.
Angiogenesis Promoters
• Placental Growth Factor. Placental growth factor is structurally related to VEGF and is also a ligand for VEGF receptor-1. PlGF is involved in a wide range of physiological processes,45,46 including recruitment of endothelial progenitor cells18 and modulation of immune system responses.47 In the setting of inflammation and ischemia, PlGF has been implicated in pathological angiogenesis,48 including laser-induced CNV.49 PlGF's role in ocular angiogenesis may involve recruitment of monocytes,19 which appear to be essential for maximal induction of ischemia-induced ocular neovascularization.50 VEGF-TRAP, a fusion protein containing both VEGF receptor 1 (R1) and VEGFR2 binding elements, and which correspondingly targets both VEGF and PlGF, is in Phase III trials (NCT00509795, Regeneron; NCT 00637377, Bayer) for neovascular AMD.
• Nicotinic Acetylcholine Receptors. Although nicotinic acetylcholine receptors are known principally as the mediators of synaptic transmission, they also appear to play a role in ocular neovascularization. In cultured cells, nicotine was found to stimulate endothelial cell proliferation, migration, and tube formation,13 as well to upregulate the expression of VEGF and VEGFR2.15 Oral administration of nicotine increased the severity of laser-induced CNV51,52; an effect mediated in part by increased recruitment of bone marrow precursor cells.53 Moreover, subcutaneous administration of mecamylamine, an inhibitor of nicotinic acetylcholine receptors, blocked CNV induction.52 Topically administered mecamylamine as a treatment neovascular AMD is in a Phase II trial (NCT00607750, CoMentis).
• Mammalian Target of Rapamycin. mTOR is a component of a heterotrimeric protein kinase that signals through two protein complexes, mTORC1 and mTORC2, to regulate a variety of processes, including angiogenesis54 and immune homeostasis.44 Rapamycin (also known as sirolimus) inhibits formation of mTORC1,54 and has been shown to both reduce VEGF expression in RPE cells12 and to inhibit various forms of ocular angiogenesis in preclinical models, including CNV as well as retinal and corneal neovascularization.5,55,56
In addition, rapamycin has been found to inhibit VEGF-induced vascular permeability.57 Palomid 529, an inhibitor of both mTORC1 and mTORC2 pathways, recently has been shown to possess similar effects both on ocular neovascularization and on VEGF-induced vascular permeability.58,59 Everolimus, another mTOR inhibitor is also being tried for the treatment of neovascular AMD (NCT00857259, Novartis).
Several clinical trials are examining the utility of rapamycin for the treatment of neovascular AMD. In an interim analysis of a Phase I/II study, intravitreal or subconjunctival administration of rapamycin was well tolerated and associated with improvements in both visual acuity and retinal thickness (NCT00712491, Macu-Sight).60
In addition, another Phase II trial is examining the use of subconjunctival rapamycin in combination with intravitreal ranibizumab (NCT00766337, Macu-Sight), while oral rapamycin is being compared to two anti-inflammatory agents, infliximab and daclizumab, given intravenously (NCT00304954, Phase II, National Eye Institute), both studies for the treatment of AMD. Finally, in addition to their potential as antiangiogenic agents, both Palomid 52961 and rapamycin62 have shown potential as neuroprotective agents; rapamycin, administered by subconjunctival injection, is being examined in a Phase I/II trial to treat geographic atrophy secondary to AMD (NCT00766649; National Eye Insti-tute).
• Platelet-Derived Growth Factor. The four PDGF species, PDGF-A through PDGF-D, occurring usually as homodimers, are ligands for their cognate receptor tyrosine kinases, PDGFR-alpha and PDGFR-beta.63 Most studies on the role of PDGF in angiogenesis have focused on PDGF-B, acting through PDGFR-beta; these have identified a central role for PDGF-B in the physiology of pericytes and smooth muscle cells (collectively known as mural cells, which provide vessel wall integrity) which surround the developing blood vessels. This signaling is essential not only for pericyte proliferation and recruitment to the vasculature,20 but also for the maintenance of blood vessel cover-age.64 In addition, PDGF-B promotes the proliferation of endothelial cells21-23 and the formation of capillary tubes.22 In the eye, either gene knockout of PDGF-B65 or administration of an inhibitor of PDGFR66 resulted in incomplete pericyte coverage of the retinal vasculature and proliferative retinopathy.
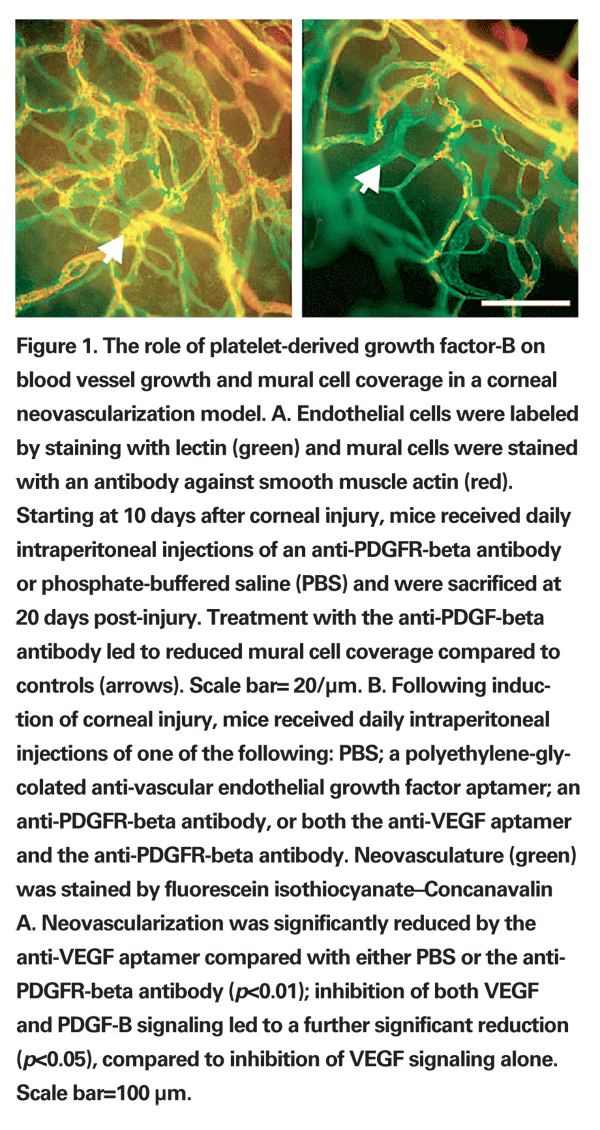
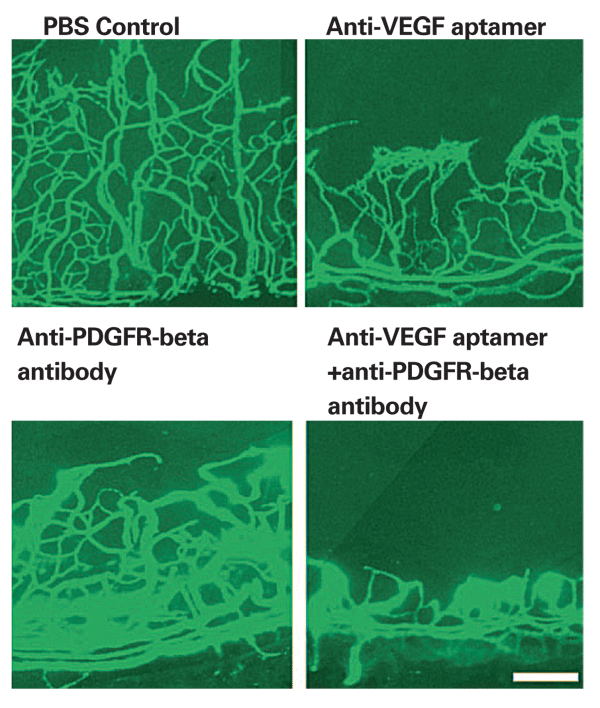
From the perspective of combinatorial approaches to therapy, it is especially relevant that that inhibition of PDGF can accentuate the impact of anti-VEGF agents. In three different mouse models of ocular neovascularization, VEGF signaling was most important for newly developing blood vessels, whereas PDGF-B inhibition affected the more mature vessels; combined VEGF and PDBF-B inhibition proved synergistic (See Figure 1).67 The drug E10030, a DNA aptamer directed against PDGF-B, is being examined in a
Phase I trial (NCT 00569140, Ophthotech) as an intravitreally administered agent, given alone or in combination with ranibizumab, as a treatment for neovascular AMD.
Preliminary results indicate that it is well-tolerated and that at three months follow-up, the majority of subjects gained three or more lines of visual acuity.68
• Tumor Necrosis Factor-alpha. TNF-alpha is a potent pro-inflammatory cytokine, now known to be involved in a number of nonimmune regulatory functions as well.24,69
These include roles in promoting angiogenesis, at least in part through upregulation of molecules such as VEGF25 and its receptor VEGFR-2,70 and PDGF-B.26 Furthermore, preclinical studies in rodent models have demonstrated the efficacy of agents targeting TNF-alpha in reducing laser-induced CNV.5,71 Of particular note, two small case series reported that intravenous injection of infliximab, a monoclonal antibody directed against TNF-alpha, provided clinical benefits for patients with neovascular AMD72 and diabetic macular edema.73 In addition, a case series in which intravitreal injections of infliximab were employed for the treatment of neovascular AMD has also been encouraging.74 The intravitreal approach is being examined in a Phase I trial in patients with neovascular AMD and DME (NCT00695682, Retina Research Foundation).
• Integrins. The integrins form a large family of membrane-bound cellular receptors, each consisting of one alpha and one beta subunit that mediate signaling through cell-cell contact or by interacting with components of the extra-cellular matrix.11 Among the many potential combinations of alpha and beta subunits, the alpha5,beta1 integrin, a key mediator in inflammatory angiogenesis,75 is of particular interest as a therapeutic target.
Small molecule inhibitors of the alpha5, beta1 integrin have demonstrated efficacy in preclinical studies of ocular neovascularization. These include JSM6247, which was found to inhibit retinal neovascularization caused by ischemia76 as well as laser-induced CNV when administered systemically77 and JSM5562, which inhibited corneal neovascularization.75 In a laser-induced CNV model in monkeys, intravitreal administration of JSM6247 produced a dose-dependent reduction in clinically significant lesions when compared to the control vehicle without any related ocular adverse effects or evidence of systemic accumulation.78 JSM6247 is in Phase I trial (NCT00536016, Jerini Ophthalmic) for neovascular AMD. In addition, volociximab, a monoclonal antibody that targets the alpha5,beta1 integrin was found to inhibit experimentally induced CNV in monkeys,79 and is also being evaluated in a Phase I trial as a treatment for neovascular AMD (NCT00782093, Ophthotech).
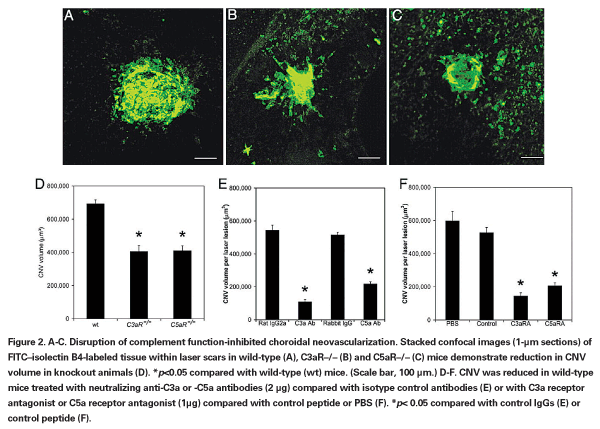
• Complement Factors. Genetic studies have identified polymorphisms of complement cascade components as risk factors for both neovascular and non-neovascular AMD80-82 and two of these, C3a and C5a, have attracted particular interest as promoters of pathological ocular neovascularization. These factors accumulate in the drusen of pa-tients with AMD, and this accumulation is also observed experimentally in mice after induction of CNV by laser wounding.7 Experimental CNV also has been found to be inhibited by deleting the genes for the receptors for C3a and C5a (See Figure 3)7 or of C3 itself,8 as well as by complement depletion with cobra venom.8 Since intravitreal injection of these factors was found to upregulate VEGF expression,7 the degree to which their induction of CNV is mediated through VEGF-independent pathways remains to be determined. A Phase I trial (NCT00709527, Ophthotech) is under way to assess the utility of ARC1905, an aptamer antagonist of C5, given either alone or in combination with ranibizumab, as a treatment for neovascular AMD; moreover, another Phase I trial is examining the effect of POT-4, an analog of the potent C3 inhibitor compstatin,83 as an intravitreal AMD treatment (NCT00473928, Potentia).
Of note, studies of the molecular mechanisms of complement have yielded information on specific regulatory components that could directly inhibit neovascularization, such as CD59. When expressed from adenovirus-expressing transgene in a mouse model system, CD59 inhibited the deposition of membrane attack complexes on the RPE.84 Furthermore, direct administration of a CD59 fusion protein, either intravitreally or intraperitoneally, significantly inhibited the induction of CNV by laser wounding, and in the converse experiment, genetic ablation of CD59 enhanced the development of CNV.84
Neuroprotectants
Geographic atrophy, a progressive degeneration of the RPE and overlying photoreceptors85,86 and potential precursor to the neovascular form of AMD, imposes a considerable burden of morbidity of the disease.87 Three approaches are being examined as potential treatments: provision of ciliary neurotrophic factor as a neuroprotectant, intravitreal administration of fenretinide, which retards the retinoid cycle, and the use of inhibitors of mTOR.
• Ciliary Neurotrophic Factor. CNTF has been shown to protect retinal neurons in a variety of animal models of retinal degeneration43 and has been developed as an intravitreal implant containing human RPE cells transfected with multiple copies of the CNTF gene.43,88 Following successful completion of a Phase I trial with patients with retinitis pigmentosa,89 the implant is now undergoing Phase II trials as a treatment for non-neovascular AMD (NCT00277134, National Eye Institute; NCT00447954, Neurotech).
• Visual cycle retardation. In conditions such as early AMD, where clearing of visual pigments may be impaired, photoreceptor damage can ensue owing to their accumulation. This destruction has been prevented in animal models by agents such as fenretinide and related compounds.90 Fenretinide is a synthetic retinoid that inhibits the normal regeneration of 11-cis-retinal, which is generated as part of the visual (retinoid) cycle.90 A Phase II trial is examining the efficacy of oral fenretinide as a treatment for geographic atrophy (NCT 00429936, Sirion).
Future Horizons
Several other angiogenic promoters and inhibitors are being evaluated as potential targets but their role is still undergoing preclinical investigation. Erythropoietin, already named for its role in promoting erythropoiesis, is also important as a survival factor for nerve cells,91 including those of the retinal pigment epithelium.92,93 Moreover, it promotes angiogenesis through recruitment of endothelial precursor cells and upregulation of VEGF,9 and both clinical94 and preclinical findings94,95 suggest that it may promote ocular neovascularization.
Several lines of evidence have implicated the renin/angiotensin system in the development of inflammatory ocular neovascularization. In the classic ren-in/an-giotensin pathway, the sequential action of renin and angiotensin converting enzyme ultimately yields angiotensin II, which then activates its Type 1 and Type 2 receptors.96 Inhibition of this pathway has been found to inhibit ocular neovascularization in several preclinical models,6,97-99 an effect that may reflect inhibition of VEGF-mediated monocyte influx.6
Finally, three additional molecular classes of interest include the Notch family of cell surface proteins, the Slit family of secreted ligands, and the endogenous inhibitor thrombospondin-1 (TSP-1). In rodent models of retinal vascular development, Notch signaling has been shown to appear as VEGF-induced sprouting 16,100; moreover, Notch-signaling also interacts with PDGF-B signaling, by upregulating synthesis of PDGFR-beta.101 Slit activates the Round-about 4 (Robo4) membrane receptor, and antagonizes VEGF-mediated actions on endothelial cells; intravitreal injection of Slit2 has been shown to inhibit pathological ocular neovascularization in murine models.39 Thrombospondin-1 (Tsp-1) has been found to inhibit endothelial cell proliferation, adhesion and migration,40 and to promote endothelial cell apoptosis.41 It is present in much lower amounts in the RPE and choriocapillaris of AMD patients than in normal controls,102 while in preclinical models it proved able to inhibit ischemia-induced neovascularization.103,104
Ongoing investigations into the mechanisms underlying the pathophysiology of AMD have provided a number of potential therapeutic strategies involving both the inhibition of angiogenesis promoters and the supplementation and/or activation of endogenous inhibitors. A number of trials are investigating the tolerability and clinical efficacy of such approaches.
Dr. Adamis is president and chief executive officer of Jerini Ophthalmic, in
For further information on clinical trials mentioned in the text, enter the cited trial number in the search field at http://clinicaltrials.gov/
1. Fiedler U, Augustin HG. Angiopoietins: A link between angiogenesis and inflammation. Trends Immunol 2006;27:552-8.
2. Thurston G, Suri C, Smith K, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 1999;286:2511-4.
3. Nambu H, Nambu R, Oshima Y, et al. Angiopoietin 1 inhibits ocular neovascularization and breakdown of the blood-retinal barrier. Gene Ther 2004;11:865-73.
4. Peters S, Cree IA, Alexander R, et al. Angiopoietin modulation of vascular endothelial growth factor: Effects on retinal endothelial cell permeability. Cytokine 2007;40:144-50.
5. Shi X, Semkova I, Muther PS, Dell S, et al. Inhibition of TNF-alpha reduces laser-induced choroidal neovascularization. Exp Eye Res 2006;83:1325-34.
6. Nagai N, Noda K, Urano T, et al. Selective suppression of pathologic, but not physiologic, retinal neovascularization by blocking the angiotensin II type 1 re-cep-tor. Invest Ophthalmol Vis Sci 2005;46:1078-84.
7. Nozaki M, Raisler BJ, Sakurai E, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A 2006;103:2328-33.
8. Bora PS, Sohn JH, Cruz JM, et al. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J Im-mu-nol 2005;174:491-7.
9. Nakano M, Satoh K, Fukumoto Y, et al. Important role of erythropoietin receptor to promote VEGF ex-pression and angiogenesis in peripheral ischemia in mice. Circ Res 2007;100:662-9.
10. Punglia RS, Lu M, Hsu J, et al. Regulation of vascular endothelial growth factor expression by insulin-like growth factor I. Diabetes 1997;46:1619-26.
11. Avraamides CJ, Garmy-Susini B, Varner JA. In-teg-rins in angiogenesis and lymphangiogenesis. Nat Rev Cancer 2008;8:604-17.
12. Stahl A, Paschek L, Martin G, et al. Rapamycin reduces VEGF expression in retinal pigment epithelium (RPE) and inhibits RPE-induced sprouting angiogenesis in vitro. FEBS Lett 2008;582:3097-102.
13. Park YJ, Lee T, Ha J, Jung IM, et al. Effect of nicotine on human umbilical vein endothelial cells migration and angiogenesis. Vascul Phar-macol 2008;49:32-6.
14. Zhang Q, Tang X, Zhang ZF, Velikina R, et al. Ni-co-tine induces hypoxia-inducible factor-1alpha expression in human lung cancer cells via nicotinic acetylcholine receptor-mediated signaling pathways. Clin Cancer Res 2007;13:4686-94.
15. Shin VY, Wu WK, Chu KM, et al. Nicotine induces cyclooxygenase-2 and vascular endothelial growth factor receptor-2 in association with tumor-associated invasion and angiogenesis in gastric cancer. Mol Cancer Res 2005;3:607-15.
16. Sainson RC, Harris AL. Regulation of angiogenesis by homotypic and heterotypic notch signaling in end-o-thelial cells and pericytes: from basic research to po-tential therapies. Angiogenesis 2008;11:41-51.
17. Wang T, Baron M, Trump D. An overview of Notch3 function in vascular smooth muscle cells. Prog Biophys Mol Biol 2008;96:499-509.
18. Li B, Sharpe EE,
19. Clauss M, Weich H, Breier G, et al. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem 1996;26:17629-34.
20. Hellstrom M, Kalen M, Lindahl P, Abramsson A, et al. Role of PDGF-B and PDGFR-beta in re-cruitment of vascular smooth muscle cells and pericytes during em-bryonic blood vessel formation in the mouse. De-vel-opment 1999;126:3047-55.
21. Beitz JG, Kim IS, Calabresi P, Frackelton AR Jr. Hu-man microvascular endothelial cells express receptors for platelet-derived growth factor. Proc Natl Acad Sci U S A 1991;88:2021-5.
22. Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, et al. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol 1994;125:917-28.
23. Marx M, Perlmutter RA, Madri JA. Modulation of platelet-derived growth factor receptor expression in microvascular endothelial cells during in vitro angiogenesis. J Clin Invest 1994;93:131-9.
24. Sethi G, Sung B, Aggarwal BB. TNF: A master switch for inflammation to cancer. Front Biosci 2008;13:5094-107.
25. Hangai M, He S, Hoffmann S, Lim JI, et al. Se-quential induction of angiogenic growth factors by TNF-alpha in choroidal endothelial cells. J Neuro-im-mu-nol 2006;171:45-56.
26. Sainson RC, Johnston DA, Chu HC, et al. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood 2008;111:4997-5007.
27. Salmi, M, Jalkanen S. A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science 1992;257:1407–9.
28. Noda K, She H, Nakazawa, T, et al. Vascular adhesion protein-1 blockade suppresses choroidal neovascularization. FASEB J 2008;22:2928-35.
29. Claesson-Welsh L, Welsh M, Ito N, et al. An-gi-o-stat-in induces endothelial cell apoptosis and activation the integrin-binding motif RGD. Proc Natl Acad Sci
30. Ramo K, Cashman SM, Kumar-Singh R. Eval-ua-tion of adenovirus-delivered human CD59 as a potential therapy for AMD in a model of human membrane attack complex formation on murine RPE. Invest Oph-thalmol
31. Dhanabal M, Volk R, Ramchandran R, Simons M, et al. Cloning, expression, and in vitro activity of hu-man endostatin. Biochem Biophys Res Commun 1999;258:345-52.
32. Campbell M, Collery R, McEvoy A, Gardiner TA, et al. Involvement of MAPKs in endostatin-mediated reg-ulation of blood-retinal barrier function. Curr Eye Res 2006;31:1033-45.
33. Zhang SX, Wang JJ, Gao G, Parke K, et al. Pig-ment epithelium-derived factor downregulates vascular endothelial growth factor expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol 2006;37:1-12.
34. Stellmach V, Crawford SE, Zhou W, Bouck N. Prevention of ischemia-induced retinopathy by the natural ocular antiangiogenic agent pigment epithelium-derived factor. Proc Natl Acad Sci U S A 2001;98:2593-7.
35. Chen L, Zhang SS, Barnstable CJ, Tombran-Tink J. PEDF induces apoptosis in human endothelial cells by activating p38 MAP kinase dependent cleavage of multiple caspases. Biochem Biophys Res Commun 2006;348:1288-95.
36. Ho TC, Chen SL, Yang YC, Liao CL, et al. PEDF induces p53-mediated apoptosis through PPAR gamma signaling in human umbilical vein endothelial cells. Cardiovasc Res 2007;76:213-23.
37. Duh EJ, Yang HS, Suzuma I, et al. Pigment epithelium-derived factor suppresses ischemia-induced retinal neovascularization and VEGF-induced migration and growth. Invest Ophthalmol Vis Sci 2002;43:821-9.
38. Yamagishi S, Abe R, Jinnouchi Y, Matsui T, et al. Pigment epithelium-derived factor inhibits vascular endothelial growth factor-induced vascular hyperpermeability both in vitro and in vivo. J Int Med Res 2007;35:896-9.
39. Jones CA, London NR, Chen H, et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med 2008;14:448-53.
40. Esemuede N, Lee T, Pierre-Paul D, Sumpio BE, et al. The role of thrombospondin-1 in human disease. J Surg Res 2004;122:135-42.
41. Nör JE, Mitra RS, Sutorik MM, Mooney DJ, et al. Thrombospondin-1 induces endothelial cell apoptosis and inhibits angiogenesis by activating the caspase death pathway. J Vasc Res 2000;37:209-18.
42. Banin E, Dorrell MI, Aguilar E, Ritter MR, et al. T2-TrpRS inhibits preretinal neovascularization and en-hances physiological vascular regrowth in OIR as as-sessed by a new method of quantification. Invest Ophthalmol Vis Sci 2006;47:2125-34.
43. MacDonald IM, Sauvé Y, Sieving PA. Preventing blindness in retinal disease: ciliary neurotrophic factor intraocular implants. Can J Ophthalmol 2007;42:399-402.
44. Weichhart T, Costantino G, Poglitsch M, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 2008;29:565-77.
45. Ribatti D. The discovery of the placental growth factor and its role in angiogenesis: a historical review. Angiogenesis 2008;11:215-21.
46. Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer 2008;8:942-56.
47. Lin YL, Liang YC, Chiang BL. Placental growth factor down-regulates type 1 T helper immune response by modulating the function of dendritic cells. J Leukoc Biol 2007;82:1473-80.
48. Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 2001;7:575-83.
49. Rakic JM, Lambert V, Devy L, et al. Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Ophthalmol Vis Sci 2003;44:3186-93.
50. Ishida S, Usui T, Yamashiro K, et al. VEGF164-me-di-ated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med 2003;198:483-9.
51. Suñer IJ, Espinosa-Heidmann DG, Marin-Castano ME, Hernandez EP, et al. Nicotine increases size and severity of experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 2004;45:311-7.
52. Kiuchi K, Matsuoka M, Wu JC, et al. Me-ca-my-la-mine suppresses Basal and nicotine-stimulated cho-roidal neovascularization. Invest Ophthalmol Vis Sci 2008;49:1705-11.
53. Hou HY, Wang YS,
54. Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 2007;12:9-22.
55. Dejneka NS, Kuroki AM, Fosnot J, Tang W, et al. Sys-temic rapamycin inhibits retinal and choroidal neo-vascularization in mice. Mol Vis 2004;10:964–72.
56. Kwon YS, Kim JC. Inhibition of corneal neovascularization by rapamycin. Exp Mol Med 2006;38:173–9.
57. Kleinman DM, et al. Si-ro-li-mus inhibits VEGF-induced microvascular hyperpermeability. Invest Ophthalmol Vis Sci 2007;48:E-Abstract 1422.
58. Sherris D, et al. Palomid 529, a Non-Steroidal Small Molecule Anti-Angiogenic Agent Inhibits Retinal and Subretinal Neo-vas-cu-lar-ization by Inhibiting the Akt/mtor Pathway. Invest -Ophthalmol Vis Sci 2008;49:E-Abstract 3766.
59. Xue Q, Hopkins B, Perruzzi C, Udayakumar D, et al. Pa-lomid 529, a novel small-molecule drug, is a TORC1/TORC2 inhibitor that reduces tumor growth, tumor angiogenesis, and vascular permeability. Cancer Res 2008;68:9551-7.
60. Dugel PU, et al. Interim Analysis of a randomized dose-escalation trial of locally-administered sirolimus to treat choroidal neovascularization secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci 2008:49;E-Abstract 582.
61. Lewis GP, et al. Palomid 529, an inhibitor of the Akt/mTOR pathway reduces photoreceptor cell death following experimental retinal detachment. Invest Ophthalmol Vis Sci 2009;50:E-Abstract 3883.
62. Pan T, Kondo S, Zhu W, Xie W, et al. Neuro-pro-tection of rapamycin in lactacystin-induced neurodegeneration via autophagy enhancement. Neu-robiol Dis 2008;32:16-25.
63. Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 2008;22:1276-312.
64. Sennino B, Falcon BL, McCauley D, et al. Se-quen-tial loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by se-lective aptamer AX102. Cancer Res 2007;67:7358-67.
65. Enge M, Bjarnegard M, Gerhardt H, et al. En-do-the-lium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. Embo J 2002;21: 4307-16.
66. Wilkinson-Berka JL, Babic S, De Gooyer T, et al. Inhibition of platelet-derived growth factor promotes pericyte loss and angiogenesis in ischemic retinopathy. Am J Pathol 2004;164:1263-73.
67. Jo N, Mailhos C, Ju M, et al. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol 2006;168:2036-53.
68. Patel S. Targeting platelet derived growth factor DB (PDGF-BB) signaling in AMD: Results from a Phase 1 trial. Presented at the Bascom Palmer Eye Institute Angiogenesis, Exudation and Degeneration meeting, Feb. 22-23, 2008, Key Biscayne,
69. Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Im-mu-no-logy 2005;115:1-20.
70. Giraudo E, Primo L, Audero E, et al. Tumor necrosis factor-alpha regulates expression of vascular endothelial growth factor receptor-2 and of its co-receptor neuropilin-1 in human vascular endothelial cells. J Biol Chem 1998;273:22128-35.
71. Olson JL, Courtney RJ, Mandava N. Intravitreal infliximab and choroidal neovascularization in an animal model. Arch Ophthalmol 2007;125:1221-4.
72. Markomichelakis NN, Theodossiadis PG, Sfikakis PP. Regression of neovascular age-related macular degeneration following infliximab therapy. Am J Ophthalmol 2005;139:537-40.
73. Sfikakis PP, Markomichelakis N, Theodossiadis GP, Grigoropoulos V, et al. Regression of sight-threatening macular edema in type 2 diabetes following treat-ment with the anti-tumor necrosis factor mono-clo-nal antibody infliximab. Diabetes Care 2005;28:445-7.
74. Theodossiadis PG, Liarakos VS, Sfikakis PP, Ver-gados IA, et al. Intravitreal administration of the anti-tumor necrosis factor agent infliximab for neovascular age-related macular degeneration. Am J Ophthalmol 2009;147:825-30.
75. Muether PS, Dell S, Kociok N, Zahn G, et al. The role of integrin alpha5beta1 in the regulation of cor-ne-al neovascularization. Exp Eye Res 2007; 85:356-65.
76. Maier AK, Kociok N, Zahn G, et al. Modulation of hypoxia-induced neovascularization by JSM6427, an integrin alpha5beta1 inhibiting molecule. Curr Eye Res 2007;32:801-12.
77. Umeda N, Kachi S, Akiyama H, et al. Suppression and regression of choroidal neovascularization by systemic administration of an alpha5beta1 integrin antagonist. Mol Pharmacol 2006;69:1820-8.
78. Zahn G, Vossmeyer D, Stragies R, et al. Preclinical Evaluation of the Novel Small Molecule Integrin ·5‚1 Inhibitor JSM6427 in Monkey and Rabbit Models of Choroidal Neovascularization. Arch Ophthalmol 2009: in press.
79. Ramakrishnan V, Bhaskar V, Law DA, et al. Pre-clin-ical evaluation of an anti-alpha5beta1 integrin antibody as a novel anti-angiogenic agent. J Exp Ther Oncol 2006;5:273-86.
80. Seddon JM, Francis PJ, George S, Schultz DW, et al. Association of CFH Y402H and LOC387715 A69S with progression of age-related macular degeneration. JAMA 2007;297:1793-800.
82.
83. Ricklin D, Lambris JD. Compstatin: a complement inhibitor on its way to clinical application. Adv Exp Med Biol 2008;632:273-92.
84.
85. Ambati J, Ambati BK, Yoo SH, Ianchulev S, et al. Age-related macular degeneration: Etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol 2003;48:257-93.
86. Cook HL, Patel PJ, Tufail A. Age-related macular degeneration: diagnosis and management. Br Med Bull 2008;85:127-49.
87. Ferris FL, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol 1984;102:1640-42.
88. Emerich DF, Thanos CG. NT-501: An ophthalmic implant of polymer-encapsulated ciliary neurotrophic factor-producing cells. Curr Opin Mol Ther 2008;10:506-15.
89. Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A 2006;103:3896-3901.
90. Maeda A, Maeda T, Golczak M, et al. Effects of potent inhibitors of the retinoid cycle on visual function and photoreceptor protection from light damage in mice. Mol Pharmacol 2006;70:1220-9.
91. Bartesaghi S, Marinovich M, Corsini E, Galli CL, et al. Erythropoietin: A novel neuroprotective cytokine. Neurotoxicology 2005;26:923-8.
92. Gawad AE, Schlichting L, Strauß O, Zeitz O. Antiapoptotic properties of erythropoietin: novel strategies for protection of retinal pigment epithelial cells. Eye 2009; Jan 16: Epub ahead of print.
93. Wang ZY, Shen LJ, Tu LL, et al. Erythropoietin protects retinal pigment epithelial cells from oxidative damage. Free Radic Biol Med 2008;Dec 24: Epub ahead of print.
94. Watanabe D, Suzuma K, Matsui S, et al. Eryth-ro-poietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med 2005;353:782-92.
95. Chen J, Connor KM, Aderman CM, Willett KL, et al. Erythropoietin siRNA suppresses retinal neovascularization in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci 2009;50:1329-35.
96. Wilkinson-Berka JL. Prorenin and the (pro)renin receptor in ocular pathology. Am J Pathol 2008;173:1591-4.
97. Nagai N, Oike Y, Izumi-Nagai K, et al. Angiotensin II type 1 receptor-mediated inflammation is required for choroidal neovascularization. Arterioscler Thromb Vasc Biol 2006;26:2252-9.
98. Nagai N, Oike Y, Izumi-Nagai K, et al. Suppression of choroidal neovascularization by inhibiting an-gi-o-tensin-converting enzyme: minimal role of bradykinin. Invest Ophthalmol Vis Sci 2007;48:2321-6.
99. Usui T, Sugisaki K, Iriyama A, et al. Inhibition of corneal neovascularization by blocking the an-gi-o-tensin II type 1 receptor. Invest Ophthalmol Vis Sci 2008;49:4370-6.
100. Siekmann AF, Covassin L,
101. Jin YH, Kim H, Oh M, Ki H, Kim K. Regulation of Notch1/NICD and Hes1 expressions by GSK-3 al-pha/- beta. Mol Cells 2008;27:Epub ahead of print.
102. Bhutto IA, Uno K, Merges C, Zhang L, et al. Re-duction of endogenous angiogenesis inhibitors in Bruch's membrane of the submacular region in eyes with age-related macular degeneration. Arch Oph-thalmol 2008;126:670-8.
103. Shafiee A, Penn JS, Krutzsch HC, Inman JK, et al. Inhibition of retinal angiogenesis by peptides derived from thrombospondin-1. Invest Ophthalmol Vis Sci 2000;41:2378-88.
104. Wu Z, Wang S, Sorenson CM, Sheibani N. At-ten-uation of retinal vascular development and neovascularization in transgenic mice over-expressing thrombospondin-1 in the lens. Dev Dyn 2006;235:1908-20.