Drug discovery isn't what it used to be. Gone are the days when pharmaceuticals operated solely on the linear development paradigm that started with new compound identification and ended with Food and Drug Administration approval. Today there are many routes leading to new medicines, including reformulations, expanded labels or new indications (repurposing), and the traditional "new molecular entity" submissions. Each of these strategies has strengths and limitations, and each provides an opportunity to expand the practitioner's therapeutic armamentarium.
In 2008, the FDA's Center for Drug Evaluation and Research approved a total of 89 new drugs, including five designated for ocular use.1 Of these, 24 were new molecular entities and only one NME, difluprednate, was a drug with an ophthalmic indication. These numbers are representative of recent trends, in which 50 to 75 percent of drug approvals are reformulations and expanded labels. This reality is due to both the high cost of NME development and to the ability of drug combinations or reformulations to deliver genuine improvements in therapeutic efficacy and safety. In reviewing the roster of recent FDA approvals (See Table 1), it's clear the drug development landscape balances the practical and the ideal.
Getting Better All the Time: Reformulations
Reformulations are changes in a drug's "molecular formulary," including any combination of changes in active ingredient concentrations, inactive components, or changes in the means of drug delivery. When drugs that have been FDA-approved are reformulated, the data used for the original approval can often be used to support the new application (examples appear in Table 1). In this way reformulation can represent a shortcut past some regulatory hurdles faced by drugs classified as NMEs. While patent protection is certainly a factor in the prevalence of reformulations, improvements in therapeutic efficacy are the overriding motivator. Often these improvements are the result of knowledge of a drug's properties gained following approval, when the number of patients (and physicians) with first-hand experience grows from clinical-trial levels to tens of millions.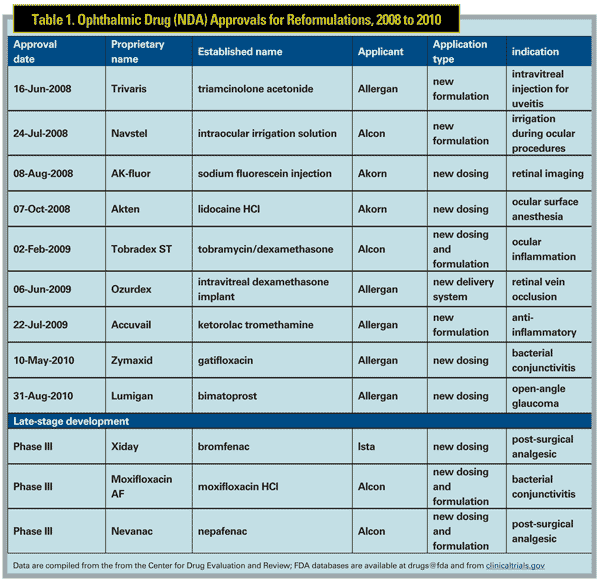
When a reformulation involves a change in active-ingredient dosing, it is typically a result of new data showing improved efficacy (usually with higher doses), reduced side effects (with lower doses) or new indications that require a dosing change relative to the original indication. For example, the recent approval of a lower-dose bimatoprost (0.01%)2 stems from studies showing that the original 0.03% formulation and the lower dose had similar efficacy. In this case, the goal of reformulation is a reduction in adverse events (such as conjunctival hyperemia) that are likely dose-dependent. Of note, the lower-dose bimatoprost formulation includes an increase in the concentration of the preservative benzalkonium chloride (0.05% to 0.2%). Another example of this type of reformulation is the fluoroquinolone gatifloxacin; the new product has an increase in the active concentration (0.5% vs. 0.3%) that provides greater efficacy or duration of antibacterial action without increasing adverse effects.
Combinations Improve Treatment
Another type of reformulation is exemplified by the drug combination of tobramycin and dexamethasone (Tobradex, Alcon) that was originally approved by the FDA in 1988. The change in formulation involves a decrease in the amount of steroid (from 0.1% to 0.05%) and the addition of an inactive agent (xanthan gum), added to stabilize the combination in a way that delivers more of each drug to the eye. The overall effect is an enhancement of both the anti-inflammatory and the antibacterial action relative to the original formulation.3 This combination drug is used for any ocular inflammation that's associated with potential secondary bacterial infection; the steroid acts to treat the inflammatory condition while the antibiotic prevents a secondary infection. The reformulation,
The Tobradex reformulation was designed to improve efficacy for an existing indication by enhancing active and inactive ingredients. In some cases the alteration of inactive ingredients is designed to target a different patient population or improve upon an existing medication in other ways. Two examples of this are the non-steroidal anti-inflammatory drug ketorolac tromethamine (Acuvail, Allergan) and the dry-eye medication Systane Balance (Alcon). Acuvail has the same active ingredient as another Allergan NSAID, but it's formulated without the preservative benzalkonium chloride.5
Systane products are designed for dry eye, and as a result of adding an additional lubricant Systane Balance is designed to treat patients with dry eye due to meibomian gland dysfunction, according to a presentation at the 2010 meeting of the Tear Film and Ocular Surface Society by University of Louisville ophthalmologist Gary Foulks, MD.
New Delivery Methods
New drug delivery modalities, especially those made possible by advances in nanotechnology, provide an opportunity to enhance therapy by improving the pharmacokinetics of an existing drug. Several recently approved or late-stage development products highlight the approaches to drug innovation that are available. Not surprisingly, these ideas take full advantage of the unique aspects of ocular anatomy to target drug delivery.
Intravitreal implants provide a sustained-release vehicle for drug delivery to areas where access via the topical route is limited. An example of this is the intravitreal dexamethasone implant (Ozurdex, Allergan), a sustained-release device for drug delivery to the posterior of the eye.6 In addition to overcoming the limits of topical administration, intravitreal delivery also reduces the systemic side effects associated with oral drugs. Implants are made of a porous polymer that slows release of drug from within the implant matrix and also allows for use of a drug with a short systemic half-life (for example, dexamethasone has a half-life in plasma of about four hours). In addition, newer implants are made with a matrix composed of carbohydrate-based polymers that break down and eventually dissolve over several months.
Implants aren't the only new approach to drug delivery. A Massachusetts biotech company, Eyegate Pharmaceuticals, is in Phase-III development for an iontophoretic drug delivery system for treatment of dry eye and uveitis.7 Like an implant, this system is designed to circumvent the physical barrier the eye presents to topical drug delivery by "pushing" drug into the eye with an applied electric field.8 Another novel delivery system in late-phase development by Vistakon is a contact lens containing ketotifen, a drug-device combination that's designed to treat allergic conjunctivitis in patients who wear corrective contact lenses.9 Often contact lens wearers avoid the use of lenses during allergy season since the use of most topical anti-allergics is contraindicated when wearing lenses. Patients must wait at least 10 minutes following eyedrop instillation before inserting contacts, a delay that leads to reduced compliance. The drug/device combination allows patients to use contacts year-round, and also provides a simplified way to deliver an anti-allergy drug when needed.
New Molecular Entities
Despite the high hurdles inherent in the drug development process, NMEs remain the gold standard of pharmaceutical development progress. As in the case of reformulations, there are variations on the theme of NME. For example, among the new drugs approved in 2009 that the Center for Drug Evaluation and Research classified as NME was bepotastine besilate (Bepreve, Ista Pharmaceuticals),10 a compound that, while new to the market in the United States, has been sold in Japan for years. As with reformulation, approval and use in another market constitutes a track record that can provide a smoother path to the
Another newly approved ophthalmic is besifloxacin (Besivance, Bausch + Lomb) a fluoroquinolone antibiotic with improved activity against gram positive bacterial strains relative to the second-generation fluoroquinolone ofloxacin.11 Besifloxacin is the first new fluoroquinolone approved for use in humans by the FDA since gatifloxacin was approved in 1999.12 As a fourth-generation fluoroquinolone, it represents the classic drug development approach of building a better molecular entity based upon an existing compound.
Perhaps the most difficult path in bringing a drug to market occurs when a molecule outside of existing previously established "compound families" is developed and brought to market. An example of this is the recently approved antihistamine alcaf-ta-dine (Lastacaft, Vistakon).13 At first glance, alcaftadine exhibits a spectrum of activities similar to medications ap-proved for allergic conjunctivitis, but it also displays the property that makes NMEs of value to the overall goal of therapeutic progress: a unique mechanism of action.
Two features of the alcaftadine mechanism of action distinguish it from other drugs for allergic conjunctivitis. First, it's a mixed-spectrum antihistamine that can act as an antagonist for H1, H2 and H4 histamine receptor isoforms;13 each of these receptors has been implicated in the underlying etiology of inflammation and allergy.14 Second, alcaftadine has anti-inflammatory properties that appear to be related to its ability to stabilize the conjunctival epithelial tight junctions.13 This effect is consistent with a number of recent studies that show a connection between disruption or breakdown of tight junction complexes15,16 and the delayed response to allergens that's a hallmark of seasonal allergic conjunctivitis and rhinitis. In a process that involves a small, stepwise progression, development of drugs with distinct mechanisms of action are the giant leaps that are essential for maintaining the momentum of clinical advancement.
As long as drug companies remain for-profit enterprises, reformulation and rebranding will play a major role in new products. Rather than a shell game, however, reformulation—especially with new delivery methods and new drug combinations—can be a critical component of the development process. When combined with more traditional routes of discovery, reformulation can provide a significant avenue to the goal of therapeutic progress.
Dr. Abelson, an associate clinical professor of ophthalmology at
1.
2. US FDA, Center for Drug Evaluation and Review, NDA approval for Lumigan. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm/lumigan. Accessed September 14, 2010.
3. US FDA, Center for Drug Evaluation and Review, NDA approval for Tobradex ST. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm/Tobradex
4. A Study to Evaluate the Clinical Efficacy and Safety of
5. US FDA, Center for Drug Evaluation and Review, NDA approval for Acuvail.
Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm/acuvail. Accessed September 14, 2010.
6. US FDA, Center for Drug Evaluation and Review, NDA approval for Ozurdex. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm/ozurdex. Accessed September 14, 2010.
7. Safety and Efficacy Study of Iontophoresis and Dexamethasone Phosphate to Treat Dry Eye. Available at: http://clinicaltrials.gov/ct2/results?term=egp-437. Accessed September 14, 2010.
8. Dixit N, Bali V, Baboota S, Ahuja A, Ali J. Iontophoresis—an approach for controlled drug delivery: A review. Curr Drug Deliv 2007;4:1:1-10.
9. Evaluation of Efficacy and Safety of an Anti-Allergy Drug With a Contact Lens in the Treatment of Allergic Conjunctivitis. Available at: http://clinicaltrials.gov/ct2/results?term=ketotifen+lens.
Accessed September 14, 2010.
10. US FDA, Center for Drug Evaluation and Review, NDA approval for bepreve. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm/bepreve. Accessed September 14, 2010.
11. US FDA, Center for Drug Evaluation and Review, NDA approval for besivance. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm/besivance. Accessed September 14, 2010.
12.
13. US FDA, Center for Drug Evaluation and Review, NDA approval for alcaftadine. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022134s000PharmR.pdf. Accessed September 14, 2010.
14. Jutel M, Akdis M, Akdis CA. Histamine, histamine receptors and their role in immune pathology. Clinical & Experimental Allergy 2009; 39:1786–1800.
15. Hughes JL, Lackie PM, Wilson SJ, Church MK, McGill JI. Reduced structural proteins in the conjunctival epithelium in allergic eye disease. Allergy 2006;61:1268-74.
16. Runswick S, Mitchell T, Davies P, Robinson C, Garrod DR. Pollen proteolytic enzymes degrade tight junctions. Respirology 2007;12:834-42.



