With the regular use of femtosecond lasers for cataract surgery on the horizon, expectations for outcomes have soared. Ultimately, it is up to the surgeon to do whatever he feels is necessary to increase the likelihood of a successful, sequelae-free surgery through the medicines he prescribes. With the trend toward off-label use of nonsteroidal anti-inflammatory drugs for the prevention of cystoid macular edema after cataract surgery, it’s crucial to keep up-to-date on the supporting studies. Here’s a look at key NSAID data.
CME and Off-label Use of NSAIDs
CME is generally characterized by the collection of fluid in the macula
and most often associated with inflammation following cataract surgery
and secondary to a breakdown of the blood-retinal barrier.1 It’s is the
main source of visual deterioration following uncomplicated cataract
surgery.1,2 The precise cause of CME is unclear. A recently conducted
review of 139,759 Medicare cataract surgeries suggested that the
overall incidence of CME is 1. 95 percent.3 Although physicians may
take prophylactic measures, the potential for CME is still an ongoing
struggle despite its relatively low incidence.
NSAIDs are cyclo-oxygenase inhibitors that work by preventing the
synthesis of prostaglandins, metabolic products of arachidonic acid,
which are potent mediators of inflammation. Thus, topically applied
ophthalmic NSAIDs are often used in the management of postop ocular
inflammation in the belief that they will hamper potential
complications and prevent CME.4 The appeal of using NSAIDs in the
treatment of ocular inflammation revolves around avoiding the recurrent
complications associated with corticosteroids, and NSAIDs’ beneficial
effects include stabilizing intraocular pressure, inducing analgesia
and reducing the risk of secondary infections. Still, it’s important to
distinguish between the approved label use and the off-label use of
NSAIDs for CME. As indicated by their labels, NSAIDs were approved to
treat inflammation, and in some cases, received a second indication for
pain after cataract surgery. This was based on the treatment of iritis.
It was the hope that despite the anatomically different locations of
iritis and CME, NSAIDs could suppress inflammation.5
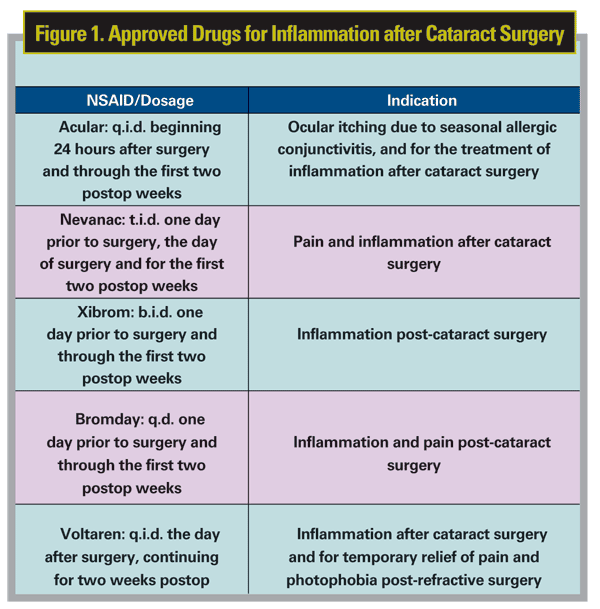 The relatively low incidence rate of CME makes it a difficult
indication for a drug to garner, so it’s common for physicians to use
drugs off-label to treat it. Presently, there is no Food and Drug
Administration-approved therapy for the treatment of CME, but there are
six topically administered ophthalmic NSAIDs with approval for the
treatment of postop inflammation in patients who have undergone
cataract extraction: Voltaren (diclofenac 0.1%); Acular (ketorolac
0.5%); Xibrom (bromfenac 0.09%); Nevanac (nepafenac 0.1%); Acuvail
(ketorolac 0. 45%) and Bromday (bromfenac 0.09%) (See Figure 1). These
drugs are routinely used to decrease inflammation that may cause CME.
The relatively low incidence rate of CME makes it a difficult
indication for a drug to garner, so it’s common for physicians to use
drugs off-label to treat it. Presently, there is no Food and Drug
Administration-approved therapy for the treatment of CME, but there are
six topically administered ophthalmic NSAIDs with approval for the
treatment of postop inflammation in patients who have undergone
cataract extraction: Voltaren (diclofenac 0.1%); Acular (ketorolac
0.5%); Xibrom (bromfenac 0.09%); Nevanac (nepafenac 0.1%); Acuvail
(ketorolac 0. 45%) and Bromday (bromfenac 0.09%) (See Figure 1). These
drugs are routinely used to decrease inflammation that may cause CME.
In addition to the approved uses, it’s become the standard of care to
use these preparations both pre- and postop to try to avoid and
possibly treat CME.6 Currently, it seems that prevention is the most
viable therapeutic option. For example, one study comparing an NSAID
(nepafenac 0.1%) with a placebo dosed q.d., b.i.d. or t.i.d. preop, the
day of surgery and postop, demonstrated a significant reduction in the
percentage of treatment failures with the NSAID compared to the placebo
(p.0.0020). Nepafenac treatment also increased the proportion of
patients with resolved ocular inflammation.7
Off-label Treatment
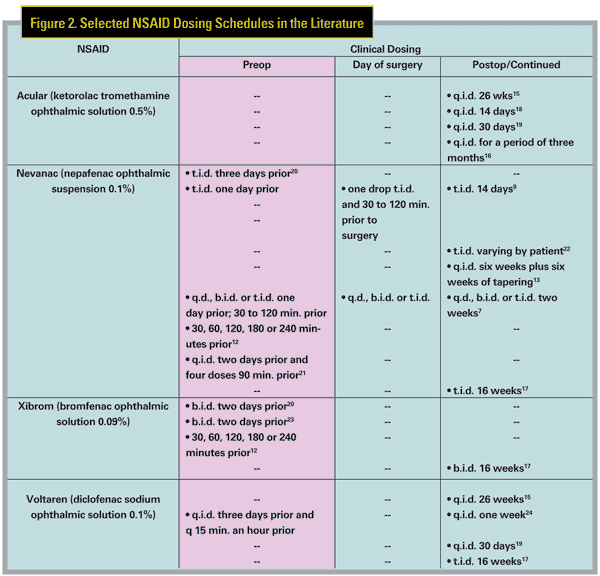
Since it's become customary to prescribe NSAIDs off-label for CME, it
should also be routine for practitioners to be conscious of outcomes,
including those of various dosing regimens (Some of them appear in
Figure 2) . CME often resolves spontaneously, and medical treatment is
actually successful in about 90 percent of cases where it's required,8
which makes persistent cases of CME worrisome. While frequently
asymptomatic, CME can cause blurred or decreased central vision and
retinal inflammation or swelling. Though vision loss from CME is
usually temporary, it can be permanent in some cases.
Some risk factors for CME include pre-existing ocular inflammation,
epiretinal or vitreoretinal interface membrane problems, and diabetic
retinopathy.1 For patients without risk factors, preoperative dosing of
NSAIDs for one to two days is recommended and postop dosing of a drop
q. i.d. for an average of three to four weeks is typical. For patients
exhibiting any risk factors, treatment should be initiated earlier and
extended longer, and any patients with increased risk factors should
receive preop and postop dosing for several months.1
Clinical Experience
Clinical experience gives prescribing physicians the most current and
updated information regarding the pharmacokinetics, efficacy and safety
of a drug.
While the perfect study would lookat the actual incidence of CME
ratherthan a surrogate, studies often use surrogates such as retinal
thickness as endpoints for CME. One study found that adding
perioperative topical ketorolac tromethamine 0.4% to prednisolone
acetate 1%, as opposed to prednisolone alone, can provide measurable
benefits in patients without known risk factors for CME. The addition
of ketorolac 0.4% beginning three days before surgery and extending
roughly four weeks postoperatively significantly reduced the incidence
of CME, defined by biomicroscopy and optical coherence tomography
analysis as “definite,” “probable” or “possible,” and associated with
retinal thickness. No patients in the ketorolac and steroid group had
clinically apparent CME, in contrast to five patients in the steroid
group alone (p=0.032). Furthermore, based on OCT, no patients in the
ketorolac and steroid group had definite or probable CME, compared with
six steroid patients (2.4 percent; p=0.018).10 Although this study
assesses the effects of extended perioperative ketorolac with steroids
as CME prophylaxis for uncomplicated cataract surgery, it can be argued
that the primary endpoint of studies evaluating CME should be visual
function.11
Ocular instillation of topical NSAIDs delivers ocular tissue and
aqueous humor levels of the drugs adequate to impede prostaglandin
synthesis,4 which is crucial in order to create therapeutic
anti-inflammatory effects in the target areas. One study examined the
aqueous humor concentrations and cyclo-oxygenase inhibitory activities
of a drop of nepafenac 0.1%, ketorolac 0.4% or bromfenac 0. 09% 30,
60, 120, 180 or 240 minutes before cataract surgery. Nepafenac had the
shortest time to peak concentration and the greatest peak aqueous humor
concentration compared to the other drugs (C[max]). Ketorolac showed
the most potent COX-1 inhibition,and nepafenac showed significantly
greater ocular bioavailability.12
 The peak incidence of CME after surgery varies between four and six
weeks,1 though in some cases its onset may be delayed for months. Even
so, studies have shown the promising effects of NSAIDs in the treatment
of established, chronic CME. In one study, known steroid responders
with CME were treated with nepafenac 0. 1% and experienced significant
improvement in visual acuity and retinal thickness at four and 12 weeks
post-treatment compared with baseline (p<0.0001), possibly due to
the penetration ability of nepafenac.1,13,14 Another study concluded
that diclofenac sodium 0.1% was as effective as topical ketorolac
tromethamine 0. 5% solution in reducing the severity and duration of
clinical CME after uneventful cataract surgery.15 After observing for
improvement in CME and visual acuity, within 26 weeks diclofenac
eliminated CME in 14 Patients (78 percent) and ketorolac eliminated it
in 12 patients (75 percent) (p=0.86, CI 95%). It’s important to note,
however, that studies conducted without a placebo make it difficult to
tell the true effect beyond the natural healing response of the eye.
The peak incidence of CME after surgery varies between four and six
weeks,1 though in some cases its onset may be delayed for months. Even
so, studies have shown the promising effects of NSAIDs in the treatment
of established, chronic CME. In one study, known steroid responders
with CME were treated with nepafenac 0. 1% and experienced significant
improvement in visual acuity and retinal thickness at four and 12 weeks
post-treatment compared with baseline (p<0.0001), possibly due to
the penetration ability of nepafenac.1,13,14 Another study concluded
that diclofenac sodium 0.1% was as effective as topical ketorolac
tromethamine 0. 5% solution in reducing the severity and duration of
clinical CME after uneventful cataract surgery.15 After observing for
improvement in CME and visual acuity, within 26 weeks diclofenac
eliminated CME in 14 Patients (78 percent) and ketorolac eliminated it
in 12 patients (75 percent) (p=0.86, CI 95%). It’s important to note,
however, that studies conducted without a placebo make it difficult to
tell the true effect beyond the natural healing response of the eye.
Another study looked at patients with pseudophakic CME diagnosed more
than two years after cataract surgery and the effectiveness of
ketorolac tromethamine 0.5% dosed q.i.d. for at least three months and
continued until the CME resolved.16 Best corrected visual acuities
measured on retroilluminated Bailey-Lovie charts and contact lens
biomicroscopy were performed at each visit, and fluorescein angiography
was performed at the initial visit, the first three-month follow-up
visit and as clinically indicated thereafter. Although some patients
did experience a recurrence of CME and a reduction in visual acuity,
four out of five of these patients responded favorably when restarted
with ketorolac. While this study shows an effectiveness of ketorolac on
established CME, it also suggests that persistent use might be required.
The surgeon’s experience will determine the best treatment. When
patients present with extensive inflammation after surgery, we may find
that they’re better treated with a potent topical steroid dosed eight
times a day to target the inflammation, and may see results sooner, in
some cases as soon as two weeks. Newer, potent steroids allow
clinicians to treat this way.
Conventional observations from cataract surgeons and a review of the
literature1,6 suggest that the length of use for ophthalmic NSAIDs as
monotherapy or combination therapy in conjunction with corticosteroids
varies based on risk factors for CME. One study examined the use of
topical NSAIDs (diclofenac 0.1%, ketorolac 0. 4%, nepafenac 0.1%,
bromfenac 0. 09%) or placebo with intravitreal steroids and
antiangiogenesis treatment for chronic pseudophakic CME. The results
concluded that NSAID therapy assisted the improvements produced by
steroids and antivascular endothelial growth factor therapy. Nepafenac-
and bromfenac-treated eyes compared with placebo showed reduced retinal
thickness at 12 and 16 weeks (nepafenac, p=0.0048, brom fenac,
p=0.0113). Nepafenac produced a sustained improvement in visual acuity
at weeks 12 and 16.17 This regimen shows potential for patients
unresponsive to less-invasive therapies.
Reading the Literature
Prescribing off-label dosing is a must in the ophthalmic world, but
constant, continuing self-education must accompany it. Also, it’s
essential that patients complete the entire course of treatment, even
if symptoms seem to have resolved. In the end, prescribing physicians
must be aware of all peer-reviewed literature pertaining to CME and
NSAIDs. While offlabel use is still caveat emptor, there are dedicated
ophthalmologists at both the FDA and at drug companies working together
toward the approval of indications, and to ensure that drugs pass
rigorous scientific and regulatory requirements.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. O'Brien TP. Emerging guidelines for use of NSAID therapy to optimize cataract surgery patient care. Curr Med Res Opin 2005;21:7:1131-1137.
2. Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc 1998;96:557.
3. Schmier JK,
4. Flach AJ. Topical nonsteroidal antiinflammatory drugs in ophthalmology. Int Ophthalmol Clin 2002;42:1:1-11.
5. Hsu JK, Johnston WT, Read RW, et al. Histopathology of corneal melting associated with diclofenac use after refractive surgery. J Cataract Refract Surg 2003;29:2:250-256.
6. Colin J. The role of NSAIDs in the management of postoperative ophthalmic inflammation. Drugs 2007;67:9:1291-1308.
7. Maxwell WA, Reiser HJ, Stewart RH, et al. Nepafenac dosing frequency for ocular pain and inflammation associated with cataract surgery. J Ocul Pharmacol Ther 2008;24:6:593-599.
8. Wolfensberger TJ, Herbort CP. Treatment of cystoid macular edema with non-steroidal anti-inflammatory drugs and corticosteroids. Doc Ophthalmol 1999;97:3-4:381-386.
9. Lane SS, Modi SS, Lehmann RP, et al. Nepafenac ophthalmic suspension 0.1% for the prevention and treatment of ocular inflammation associated with cataract surgery. J Cataract Refract Surg 2007;33:1:53-58.
10. Wittpenn JR, Silverstein S, Heier J, et al. A randomized, masked comparison of topical ketorolac 0.4% plus steroid vs. steroid alone in low-risk cataract surgery patients. Am J Ophthalmol 2008;146:4:554-560.
11. Kim A, Stark WJ. Are topical NSAIDs needed for routine cataract surgery? Am J Ophthalmol 2008;146:4:483-485.
12. Walters T, Raizman M, Ernest P, et al. In vivo pharmacokinetics and in vitro pharmacodynamics of nepafenac, amfenac, ketorolac, and bromfenac. J Cataract Refract Surg. 2007;33:9:1539-1545.
13.
14. Lindstrom R, Kim T. An examination of data and expert opinion on the clinical utility of nepafenac. Curr Med Res Opin 2006;22:2:397-404.
15.
16. Weisz JM,
17.
18. Solomon KD, Cheetham JK, DeGryse R, Brint SF, Rosenthal A. Topical ketorolac tromethamine 0.5% ophthalmic solution in ocular inflammation after cataract surgery. Ophthalmology 2001;108:2:331-337.
19. Flach AJ, Dolan BJ, Donahue ME, et al. Comparative effects of ketorolac 0.5% or diclofenac 0.1% ophthalmic solutions on inflammation after cataract surgery. Ophthalmology 1998;105:9:1775.
20. Heier JS, Awh CC, Busbee BG, et al. Vitreous nonsteroidal antiinflammatory drug concentrations and prostaglandin E2 levels in vitrectomy patients treated with ketorolac 0.4%, bromfenac 0.09%, and nepafenac 0.1%. Retina 2009;29:9:1310-1313.
21. Bucci FA, Jr.,
22. Hariprasad SM, Callanan D, Gainey S,et al. Cystoid and diabetic macular edema treated with nepafenac 0.1%. J Ocul Pharmacol Ther 2007;23:6:585-590.
23. Bucci FA, Jr., Waterbury LD. Aqueous prostaglandin E(2) of cataract patients at trough ketorolac and bromfenac levels after 2 days dosing. Adv Ther 2009;26:6:645-650.
24. Roberts CW. Pretreatment with topical diclofenac sodium to decrease postoperative inflammation. Ophthalmology 1996;103:4:636-639.



