Pre-assembled with a standard infusion sleeve, the coaxial handpiece is designed to work with the Stellaris and Stellaris PC systems for lens removal using an I/A mode. The handpiece is used as a replacement for the ultrasound phacoemulsification handpiece and is green-colored to avoid confusion with the I/A handpiece for cortical cleanup. In addition to the standard incision format (for incision sizes greater than 2.4 mm), the ZeroPhaco I/A handpiece is also available for use in a MICS 2.2mm incision as well.
The ZeroPhaco I/A handpiece joins a complete family of purpose-designed, single-use Bausch + Lomb instruments for the rest of the femtocataract procedure, including fixation forceps, lens manipulators and lid speculum.
For information, visit bausch.com.
Widefield OCT Imaging from Heidelberg Engineering
Heidelberg Engineering GmbH has announced a new widefield OCT imaging modality for its Spectralis OCT family of products.
 |
Until recently, the area scanned during an examination has been limited to a 30-degree field of view. The new Widefield OCT module for the Spectralis expands the OCT field of view to 55 degrees. The new Widefield OCT module allows imaging of the macula, the optic nerve head and the periphery in one comprehensive examination.
The Widefield OCT Module consists of a 55-degree widefield lens and dedicated software to acquire and process the expanded scan patterns. Customers who already own a widefield lens for Spectralis can simply purchase a software upgrade to utilize this new modality.
The Widefield OCT scans can be acquired simultaneous to all confocal laser scanning laser fundus imaging modalities available on the various models of the Spectralis product family. Widefield OCT is another module offered by the flexible and expandable OCT imaging platform of Spectralis.
For information, visit heidelbergengineering.com.
iScan for Simplified OCT
 |
The iScan system simplifies optical coherence tomography technology, making it accessible to a range of eye-care professionals in any practice setting. With Optovue’s proprietary iWellness scan, iScan guides the patient through the entire exam and requires minimal operator involvement. The iWellness scan provides a cross-sectional view of the retinal layers, a retinal thickness map and a ganglion cell complex map. These outputs enable the doctor to identify signs of disease or otherwise confirm a patient’s ocular health.
iScan is being introduced at a very attractive price point, and the revenue generated from the iWellnessExam delivers exceptional return on investment. For information, visit optovue.com.
Rayner Debuts RaySert PLUS
Rayner Intraocular Lenses Ltd. has announced the launch of the company’s new injector, RaySert PLUS in the U.S. market after receiving 510(k) clearance from the Food and Drug Administration.
RaySert PLUS is designed for safe and effective implantation of the C-flex Aspheric IOL with a simple and controlled IOL delivery through a wound-assisted 2.2-mm clear cornea mini-incision. For information, visit rayner.com.
|
Allergic conjunctivitis is the most common form of ocular allergy in the United States and around the world; seasonal and perennial allergies affect at least 20 percent of the population, and 70 percent to 80 percent of these patients report that their allergies include ocular symptoms.1 While current treatment strategies include artificial tears, mast cell stabilizers and corticosteroids, the combination of safety, comfort and efficacy has made topical antihistamines the first choice for pharmacotherapy of AC. Among currently approved agents, only two are approved for once-daily dosing: Pataday (0.2% olopatadine, Novartis) and Lastacaft (0.25% alcaftadine, Allergan). A newly published study, supported by Allergan, provides the first direct comparison of these two topical antihistamines, focusing on the duration of relief from signs and symptoms of allergic conjunctivitis.2
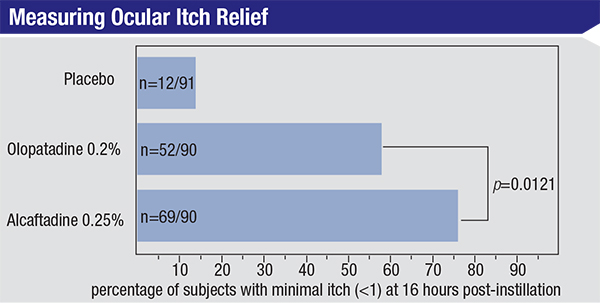
The study is an analysis of pooled data from two double masked, randomized, placebo-controlled trials that each compared olopatadine 0.2%, alcaftadine 0.25%, and a placebo treatment using the CAC (Conjunctival Allergen Challenge) model of ocular allergy. A total of 284 subjects were included in the analysis of efficacy and safety. In the pooled analysis, mean itch scores for all time points were significantly lower in alcaftadine-treated subjects compared to the subjects receiving olopatadine (0.68 vs. 0.92; p=0.039). The clinical significance of this difference was examined by a minimal itch analysis. Subjects with minimal itch were defined as those with itch scores <1 at all time points. Following an allergen challenge 16 hours after instillation of test agents, 76.1 percent of subjects in the alcaftadine group reported minimal itch, while 58.1 percent of those in the olopatadine group reported minimal itch (p=0.0121; significant by Fisher’s exact test).
Evidence from a number of studies supports the idea that chronic or severe allergy may involve long-lasting changes in the epithelial barrier of the cornea and conjunctiva, and that such changes may underlie both the symptomology of perennial allergy and the exacerbating effects of urban pollutants on allergic signs and symptoms.3,4 A preclinical study published in 2011 examined eosinophil recruitment and epithelial barrier dynamics in a murine model of chronic allergy.5 In this study, alcaftadine was also found to be superior to olopatadine in terms of its ability to reduce eosinophil infiltration and mitigate the changes in epithelial structural integrity. These effects may help to explain the differences seen between the two drugs in the pooled analysis by Eugene McLaurin and colleagues.1
 |
1. Blaiss MS. Allergic rhinoconjunctivitis: Burden of disease. Allergy Asthma Proc 2007;28:393–7.
2. McLaurin EB, Ciolino JB, Ackerman SL, et al. Ocular Itch Relief With Alcaftadine 0.25% Versus Olopatadine 0.2% in Allergic Conjunctivitis: Pooled Analysis of Two Multicenter Randomized Clinical Trials. Adv. Ther 2014; published online 27 September 2014.
3. Hughes JL, Lackie PM, Wilson SJ, Church MK, McGill JI. Reduced structural proteins in the conjunctival epithelium in allergic eye disease. Allergy 2006; 61:1268-74.
4. Runswick S, Mitchell T, Davies P, Robinson C, Garrod DR. Pollen proteolytic enzymes degrade tight junctions. Respirology 2007; 12:834-42.
5. Ono SJ, Lane K. Comparison of effects of alcaftadine and olopatadine on conjunctival epithelium and eosinophil recruitment in a murine model of allergic conjunctivitis. Drug Des Devel Ther 2011;5:77-84.



