Ocular ultrasonography depicted an echolucent, dome-shaped lesion with no extrascleral extension. The lesion was maximally visible (inflated) in superonasal gaze and not visible (deflated) in primary gaze or with digital pressure on the globe (Figure 2B). Video-capture fluorescein angiography revealed a patchy area of mild hyperfluorescence with a hypofluorescent rim that corresponded to the lesion (Figure 3A, arrows). Simultaneous video-capture indocyanine green angiography demonstrated more revealing features of early homogenous filling of the lesion superonasally without leakage or staining (Figure 3B, arrow). Gaze
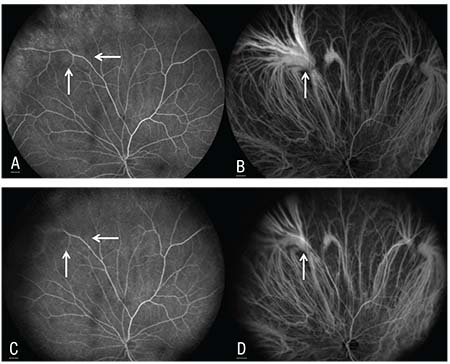 |
| Figure 3. Fluorescein angiography showing a patchy area of mild hyperfluorescence with a hypofluorescent rim (arrows) that corresponds to the lesion (A). Simultaneous indocyanine green angiography demonstrating early homogenous filling of the lesion superonasally (arrow) without leakage or staining (B). Gaze repositioning or digital pressure caused the lesion (arrows) to decrease in fluorescence on FA (C) and cyanescence on ICGA (D). |
Discussion
Uveal melanoma is the most common primary intraocular tumor in adults. One study estimated the worldwide incidence to be 7,095 cases annually, with 4,747 in Caucasian, 1,286 in Asian, 738 in Hispanic, and 316 in African patients.1 This malignancy is slightly more common in males (4.9 per million) than in females (3.7 per million).2 In an analysis of 8,033 patients with uveal melanoma over a 40-year period, Wills Eye’s Carol Shields, MD, and her colleagues reported that the mean patient age at diagnosis was 58 years, with a range of 3 to
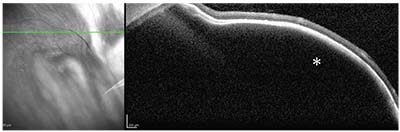 |
| Figure 4. Optical coherence tomography documenting a smooth, dome-shaped elevation of the choroid and retinal pigment epithelium-Bruch’s membrane complex (asterisk) without subretinal fluid or retinal edema. |
Several conditions can clinically simulate uveal melanoma, leading to diagnostic ambiguity.4 In an analysis of 12,000 patients referred for uveal melanoma over a 25-year period at Wills Eye Hospital, Drs. Jerry and Carol Shields’ group found that 1,739 (14 percent) had a simulating condition, namely pseudomelanoma.5 The most frequent pseudomelanomas included choroidal nevus (49 percent), peripheral exudative hemorrhagic chorioretinopathy (8 percent), congenital hypertrophy of the retinal pigment epithelium (6 percent), idiopathic hemorrhagic detachment of the retina or retinal pigment epithelium (5 percent), circumscribed choroidal hemangioma (5 percent), and age-related macular degeneration (4 percent).5 Vortex vein varix comprised only 0.4 percent of cases, likely due in part to its relatively rare recognition.5 Though this condition is benign and asymptomatic, its potential confusion for small choroidal melanoma makes the varix clinically important.
The diagnosis of vortex vein varix is facilitated by an understanding of the choroidal venous system. The choroidal blood drains from the eye through large venous tributaries that coalesce into an average of eight vortex veins, with at least one vortex vein per quadrant of the eye.6,7 These vortex veins exit the globe through scleral canals and typically merge with other vortex veins in the orbit before draining into the ophthalmic vein.6 Prior to entering the scleral canal, about half of the vortex veins have an aneurysmal dilatation of varying sizes and shapes, termed a vortex vein ampulla.7 These ampullae generally are visible anterior to the equator of the globe. In rare cases, an ampulla bulges large enough to resemble a choroidal neoplasm. In this case, the dilatation is termed a varix of the vortex vein ampulla.6
The vortex vein varix is most common in middle-aged or older patients but has been reported in patients as young as 23 years.8 This lesion is typically single and unilateral but can be multifocal or bilateral.6,8 The varix appears as a smooth, reddish-brown, subretinal elevation along the equator, usually in the superonasal or inferonasal quadrants.6,9
The vortex vein varix characteristically inflates with gaze toward the lesion, achieving a basal diameter of up to 6 mm and a thickness up to 2.5 mm.6 The enlarging varix can compress the surrounding choroid and display a brownish-red color that raises concern for choroidal melanoma.6 However, the varix deflates and the hyperpigmentation disappears with return to primary gaze or pressure on the globe. The etiology of this fluctuation is unclear, but likely involves gaze-evoked narrowing of the scleral canals or kinking of the episcleral vortex veins, leading to stagnation of venous outflow and inflation of the varix.6 A Valsalva maneuver, prone positioning and other factors that increase intraocular venous pressure have also been implicated.6,9
The dynamic nature of the varix’s size distinguishes it from choroidal melanoma, which doesn’t diminish with gaze or pressure.6 Ancillary testing can help demonstrate this distinction. Ultrasonography of the varix reveals inflation with gaze toward the lesion and deflation with primary gaze or pressure with the probe.10 OCT shows an ectatic choroidal vessel with an internal low optical signal, corresponding to the dilated ampulla.11 ICGA is particularly useful because it delineates the surrounding choroidal vasculature from the gaze- and pressure-dependent vascular tree of the varix.9 Together, these tools help distinguish the benign vortex vein varix from choroidal melanoma and prevent unnecessary medical treatment. REVIEW
1. Kivela T. The epidemiologic challenge of the most frequent eye cancer: Retinoblastoma, an issue of birth and death. Br J Ophthalmol 2009;93:1129-31.
2. Singh AD, Topham A. Incidence of uveal melanoma in the United States: 1993–1997. Ophthalmology 2003;110:956-61.
3. Shields CL, Kaliki S, Furuta M, et al. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8033 cases. Retina 2012;32:1363-72.
4. Shields CL, Manalac J, Das C, Ferguson K, Shields JA. Choroidal melanoma: Clinical features, classification, and top 10 pseudomelanomas. Curr Opin Ophthalmol 2014;25:3:177-85.
5. Shields JA, Mashayekhi A, Ra S, Shields CL. Pseudomelanomas of the posterior uveal tract. The 2006 Taylor Smith Lecture. Retina 2005;25:767-71.
6. Gündüz K, Shields CL, Shields JA. Varix of the vortex vein ampulla simulating choroidal melanoma: Report of four cases. Retina 1998;18:4:343-7.
7. Rutnin U. Fundus appearance in normal eyes. I. The choroid. Am J Ophthalmol 1967;64:5:821-39.
8. Osher RH, Abrams GW, Yarian D, Armao D. Varix of the vortex ampulla. Am J Ophthalmol 1981;92:653-60.
9. Kang HK, Beaumont PE, Chang AA. Indocyanine green angiographic features of varix of the vortex vein ampulla. Clin Exp Ophthalmol 2000;28:4:321-3.
10. Shields JA, Shields CL, Mashayekhi A, Seong RA. Pseudomelanomas of the posterior uveal tract. Retina 2005;25:6:767-71.
11. Ismail RA, Sallam A, Zambarakji HJ. Optical coherence tomographical findings in a case of varix of the vortex vein ampulla. Br J Ophthalmol 2011;95:8:1169-70.



