|
James Tsai, MD |
There is considerable debate in the literature whether normal tension glaucoma (NTG) represents a distinct entity or is simply primary open-angle glaucoma with intraocular pressure within the normal range. Since IOP is a continuous variable with no definite dividing line between normal and abnormal, some have questioned whether the term NTG is misleading.1 In our view, the definition of NTG should be based on characteristic glaucomatous optic disc cupping and associated visual field defects in eyes with intraocular pressure in the statistical norm and the absence of ocular or systemic features contributing to other forms of optic neuropathy.
Background
Normal Tension Glaucoma is a disease of elderly persons, and the mean reported age in clinical studies generally is in the 60s; myopic patients with this disease are significantly younger. On average, in population-based surveys, 30 to 40 percent of patients diagnosed with glaucomatous visual field defect have normal IOPs.2,3 In Japan, NTG accounts for the majority of patients diagnosed with primary open-angle glaucoma.4 The relatively high prevalence of NTG may in part be explained by findings that more than 50 percent of patients with glaucoma have been previously undiagnosed in population-based, cross-sectional studies.5
Patients with NTG may be asymptomatic and present when they already have extensive visual field loss. A high index of suspicion is required for the diagnosis of the disease. In patients with NTG, the optic disc rim may be significantly thinner, especially inferiorly and inferotemporally (compared to that observed in POAG patients).6 Optic disc hemorrhages and parapapillary disc atrophy (particularly in zone beta) may be more commonly observed in patients with NTG.7 Moreover, the visual field defects tend to be more focal, deeper, and closer to fixation.8 It is not unusual for a patient with NTG to present initially with a dense paracentral scotoma encroaching on fixation.
The pathogenesis of NTG remains unclear. Some have hypothesized that the presence of a progressive optic neuropathy in the context of normal IOPs suggests an underlying vascular insufficiency.9 A variety of cardiovascular and hematologic abnormalities have been described in patients with NTG including hemodynamic crisis, hypercoagulability, reduced diastolic ophthalmodynamometry, nocturnal systemic hypotension, increased blood and plasma viscosity, elevated blood cholesterol and lipids, carotid artery disease, slowed parapapillary, choroidal and retinal circulation and migraine.10 In addition, studies have suggested an association of immune-related diseases with NTG, including an increased incidence of paraproteinemia and autoantibodies.11
Clinical Evaluation
The most important part of the diagnostic workup for NTG is a careful and comprehensive ocular and systemic history and examination. Questions should be posed regarding any previous episodes of IOP elevation, ocular trauma/inflammation, use of medications (e.g., steroids), sleep-related breathing disorders (e.g., sleep apnea), and nocturnal blood pressure dips. In particular, obstructive sleep apnea may be a significant risk factor for NTG, and thus it is important to take a sleep history from patients with NTG.12
A recent study also reported that marked circadian fluctuation in mean ocular perfusion pressure (associated with nocturnal blood pressure reduction) may be a significant risk factor for the development of NTG.13 The clinician should also inquire about any history of hemodynamic crisis including episodes of blood loss, hypotension, anemia, syncope, blood transfusions and falls. Neurological symptoms such as weakness in extremities, dizziness, headache, loss of consciousness and diplopia should be noted. If elicited, the presence of neurological symptoms may indicate the need for neuro-ophthalmological evaluation to rule out neurological diseases that may masquerade as NTG.
The comprehensive ocular examination should include slit-lamp examination, color vision and pupillary reaction testing, central corneal thickness measurement, diurnal IOP assessment, gonioscopy, perimetry (if indicated), and stereoscopic optic disc examination. As an example, careful slit-lamp evaluation can rule out burned-out pigmentary glaucoma as a potential diagnosis by looking for pigment dispersion on corneal endothelium, iris transillumination defects, and increased pigmentation in the anterior chamber angle. Diurnal variations in IOP must be ruled out, since previously undetected POAG with large swings in diurnal IOP values is on the differential diagnosis.
Gonioscopy should be performed to rule out angle closure, angle recession, increased pigmentation (suggestive of pseudoexfoliation or pigmentary dispersion), or evidence of previous intraocular inflammation. Careful stereoscopic optic disc evaluation is essential to exclude other congenital or acquired disc anomalies such as optic disc coloboma, drusen and a physiologically enlarged cup. Structural evaluation of the optic disc and retinal nerve fiber layer may prove helpful for the early detection of NTG prior to the development of discernable visual field defects.
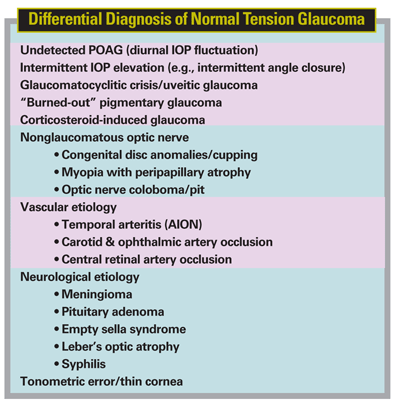
The diagnosis of NTG is made only after a comprehensive assessment has been performed (i.e., it is a diagnosis of exclusion). Confirmation of the disease requires repetitive and diurnal IOP measurements to rule out ocular hypertension and/or POAG. Acquired loss (not hereditary) of color vision may indicate a neurological condition as the cause of the optic neuropathy. The presence of a relative afferent pupillary defect may indicate a neurologic defect. Correspondence of the glaucomatous optic disc cupping/neuroretinal rim changes with the visual field defect may be reassuring for a glaucomatous process (though not always). Identification of potentially causative vascular factors (e.g., migraine, vasospasm) is important in making the diagnosis; further diagnostic workup may include measurement of the cold response of the nail fold capillaries, ambulatory blood pressure measurement to identify nocturnal dips, and also color Doppler imaging of the common carotid artery to rule out blood flow abnormalities.10 The differential diagnosis also includes neurologic lesions and conditions. A brain scan and/or additional neurological investigation may be indicated if any disparity arises between the optic disc appearance (e.g., extent of cupping, neural rim loss) and the visual field defect. The differential diagnosis is extensive and requires thoughtful consideration (See Table).
Current Treatment
The treatment for NTG has been the subject of intense debate for many years. Current treatment modalities are aimed at controlling IOP, a widely accepted risk factor in the progression of NTG. The Collaborative Normal Tension Glaucoma Study reported that aggressive IOP reduction of at least 30 percent from baseline levels reduced the extent of progressive visual field loss.14,15 In that study, therapy was initiated in patients with NTG with visual field loss threatening fixation, disc hemorrhage, and documented visual field or optic nerve progression. The study results showed that aggressive IOP reduction with medications and/or surgery decreased the risk of progression from 35 percent to 12 percent over a five-year period.14
However, a substantial percentage of the untreated patients (65 percent) did not progress, thereby demonstrating the extremely variable clinical course observed in NTG. Moreover, the favorable effect of IOP reduction on progression of visual change was only noted after the impact of cataracts on visual field progression, produced largely by surgery, was removed. Since not all untreated patients with NTG progress, the clinician must consider the natural history of the disease (as well as whether there is risk of serious visual loss) before embarking on IOP reduction therapy.15 A faster rate of progression occurs in women (risk ratio=1.85), in patients with migraine headaches (risk ratio=2.58), and in the presence of disc hemorrhages (risk ratio=2.72).16
Before initiating treatment of NTG, the clinician must first determine whether the affected patient is at risk for progression (i.e., progressive vs. non-progressive form). If the patient is judged to have the progressive form of the disease, then it is very helpful to determine a target pressure level. For example, if the untreated IOPs have been in the high teens, then a target level of 14 mmHg or less (with a minimum 30-percent reduction) seems a reasonable place to start. If the untreated IOPs have been in the mid-teens, then the clinician should aim for pressure reductions in the 10 to 12 mmHg range. Once the target IOP levels have been achieved, IOP measurements should be taken at different times of the day to rule out diurnal fluctuation. The target pressure may need to be reset (and therapy changed) if fluctuation in diurnal IOP and/or progression of optic nerves/visual fields are observed.
A common initial approach in treating NTG is medical therapy. It may be helpful to initiate a unilateral trial of the medication so that the contralateral eye can be used as a control to assess the drug's therapeutic and side-effect profile. If medical therapy is insufficient and/or intolerable in reaching the target IOP level, laser trabeculoplasty may be considered. In certain instances, glaucoma filtering surgery is indicated to obtain IOP control.17 Use of antifibrotic agents such as mitomycin-C or 5-fluorouracil may improve the overall success rate of filtration surgery.
Future Treatment
Despite apparent adequate IOP lowering, some patients still have progression of their disease. As mentioned above, a number of other risk factors besides IOP have been implicated in the pathogenesis of glaucoma (particularly NTG) including vascular, mechanical and genetic aspects, as well as myopia, endocrine abnormalities and autoimmune phenomena.18 Diurnal variations in the microcirculation of the optic nerve head may play a role in the progression of NTG in patients with vascular dysregulation, leading to both low perfusion pressure and impaired autoregulation.19 This, in turn, may lead to unstable ocular perfusion and resultant ischemia and reperfusion damage. Thus, some have advocated therapy with vasodilator drugs such as calcium-channel blockers in select NTG patients with visual field damage and vasospastic propensity.18 For example, brovincamine, a relatively selective cerebral vasodilator used to improve cerebral circulation and metabolism, has been shown to retard visual field deterioration in certain patients with NTG.20 Nimodipine, another selective calcium-channel blocker, has been reported to improve performance in visual field and color vision testing in NTG patients.21
In the future, neuroprotection therapeutic modalities will likely be considered in the treatment regimen for patients with NTG. Memantine, a potential neuroprotective agent that blocks excessive NMDA-type glutamate receptor activity without disrupting normal activity, has been approved for the treatment of Alzheimer's disease.22 Clinical studies evaluating the safety and efficacy of memantine in glaucoma are currently under way. Ginkgo biloba has also been reported to improve visual field indices in NTG patients in a prospective, randomized, placebo-controlled, double-masked crossover study.23 Thus, further research is required to improve our understanding of the pathogenesis, diagnosis, and treatment of this challenging disease.
Dr Tsai is an associate professor of ophthalmology and director of the Glaucoma Division at the Edward S. Harkness Eye Institute, Columbia University College of Physicians and Surgeons. Contact him at 212-305-9535, or jct2002@columbia.edu.
Dr. Karim is a consultant in the Glaucoma Service at Chittagong Eye Infirmary and Training Complex, Pahartali, Chittagong-4000 Bangladesh. Phone: 88-031-659018-9; e-mail: drkarim20@hotmail.com, Web: eyeinfirmary.org.
1. Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol 1991;109:1090-1095.
2. Klein BEK, Klein R, Sponsel WE, et al. Prevalence of glaucoma: The Beaver Dam eye study. Ophthalmology 1992;99:1499.
3. Dielemans I, Vingerling JR, Wolfs RCW, et al. The prevalence of primary open-angle glaucoma in a population-based study in the Netherlands: The Rotterdam study. Ophthalmology 1994;101:1851.
4. Shiose Y, Kitazawa Y, Tsukahara S, et al. Epidemiology of glaucoma in Japan: A nationwide glaucoma survey. Jpn J Ophthalmol 1991;35:133.
5. Varma R, Ying-Lai M, Francis BA, et al. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: The Los Angeles Latino Eye Study. Ophthalmology 2004;111:1439-1448.
6. Caprioli J, Spaeth GL. Comparison of the optic nerve head in high- and low-tension glaucoma. Arch Ophthalmol 1985;103:1145.
7. Tezel G, Kass MA, Kolker AE, et al. Comparative optic disc analysis in normal pressure glaucoma, primary open-angle glaucoma, and ocular hypertension. Ophthalmology 1996;103:2105.
8. Chauhan BC, Drance SM, Douglas GR, et al. Visual field damage in NTG and high-tension glaucoma. Am J Ophthalmol 1989;108:636.
9. Drance SM, Sweeney VP, Morgan RW, et al. Studies of factors involved in the production of low tension glaucoma. Arch Ophthalmol 1973;89:457.
10. Allingham RR, Damji K, Freedman S, et al. Shields' Textbook of Glaucoma, 5th edition, , Philadelphia: Lippincott Williams & Wilkins, 2005:197-216
11. Wax MB, Barrett DA, Pestronk A. Increased incidence of paraproteinemia and autoantibodies in patients with normal-pressure glaucoma. Am J Ophthalmol 1994;117:561.
12. Mojon DS, Hess CW, Goldblum D, et al. Normal-tension glaucoma is associated with sleep apnea syndrome. Ophthalmologica 2002;216:180-184.
13. Choi J, Jeong J, Cho H, Kook MS. Effect of nocturnal blood pressure reduction on circadian fluctuation of mean ocular perfusion pressure: A risk factor for normal tension glaucoma. Invest Ophth Vis Sci 2006;47:831-836.
14. Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol 1998;126:487-497.
15. Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol 1998;126:498-505.
16. Drance S, Anderson DR, Schulzer M, Collaborative Normal-Tension Glaucoma Study Group. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol 2001;131:699-708.
17. Hitchings RA, Wu J, Poinoosawmy D, McNaught A. Surgery for normal-tension glaucoma. Ophthalmology 1997;104:197-201.
18. Orgul S, Zawinka C, Gugleta K, Flammer J. Therapeutic strategies for normal-tension glaucoma. Ophthalmologica 2005;219:317-323.
19. Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 2002;21:359-393.
20. Sawada A, Kitazawa Y, Yamamoto T, et al. Prevention of visual field defect progression with brovincamine in eyes with normal-tension glaucoma. Ophthalmology 1996;103:283-288.
21. Piltz JR, Bose S, Lanchoney D. The effect of nimodipine, a centrally active calcium antagonist, on visual function and macular blood flow in patients with normal-tension glaucoma and in control subjects. J Glaucoma 1998;7:336-342.
22. Lipton SA. Possible role for memantine in protecting retinal ganglion cells from glaucomatous damage. Surv Ophthalmol 2003;48 Suppl 1:S 38-46.
23. Quaranta L, Bettelli S, Uva MG, et al. Effect of ginkgo biloba extract on preexisting visual field damage in normal tension glaucoma. Ophthalmology 2003;110:359-362.