Bausch & Lomb has launched the Crystalens Aspheric Optic (AO) Lens, which it calls the first aberration-free accommodating intraocular lens with aspheric optics, following the recent FDA approval of this newest B&L surgical product. The Crystalens AO joins the Crystalens HD and the Crystalens Five-0.
The Crystalens AO has prolate aspheric surfaces and is designed to improve retinal image quality without compromising depth of field, providing greater quality of distance and intermediate vision.
The company says that while other aspheric IOLs have negative spherical aberration, which could reduce depth of field, the Crystalens AO has zero spherical aberration, and the combination of the Crystalens platform and AO optics work together to enhance depth of field. Crystalens is the only FDA-approved accommodating intraocular lens. The Crystalens AO is composed of proprietary biosil silicone and has a thin and uninterrupted barrier edge. It will be inserted using the CI-28 injector in a controlled manner through an un-enlarged phaco incision. The Crystalens AO worldwide launch is expected to accelerate through the first quarter of 2010.
For information, visit crystalens.com or call 1 (877) See Better.
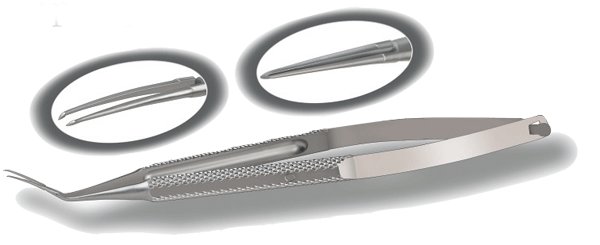
Rhein Debuts Capsulorhexis Forceps
The Imamura Capsulorhexis Cys-totome Forceps from Rhein Medical offers a host of features and benefits:
•12-mm formed blades designed to relieve stress on the incision.
• Unique direct-acting jaws able to fit through a minimal incision.
• Special cystotome tips designed to open the capsule in one maneuver.
• Micro-gap between the blades to prevent iris pickup.
Developed in coordination with Mikio Inamura, MD, PhD, the forceps are reusable and autoclaveable and are available for a 30-day surgical evaluation without obligation. For information, call (727) 209-2244.
Compulink's Advantage/Electronic Health Records Now CCHIT Certified
Compulink Business Systems an-nounced that its Advantage/EHR (electronic health record) version 10 has earned its designation as a premarket conditionally CCHIT Certified 2011 Ambulatory EHR in the Certification Commission for Health Information Technology's (CCHIT) Comprehensive certification program.
CCHIT Certified certification designates that Compulink's Advantage/EHR version 10 has been tested against the most comprehensive set of existing criteria related to items such as interoperability, privacy and security and functionality. As a premarket conditionally CCHIT Certified 2011 Ambulatory EHR, Advantage/EHR version 10 has passed inspection of 100 percent of these criteria.
In response to the new requirements of the American Recovery and Reinvestment Act of 2009 (ARRA), as well as ongoing federal rulemaking activities, the Certification Commission launched two distinct programs of certification on October 7, 2009. In addition to its established CCHIT Certified Comprehensive programs, CCHIT also launched a more limited, modular Preliminary ARRA certification program.
The CCHIT Certified 2011 Comprehensive program is differentiated from the Preliminary ARRA program by providing a rigorous, multifaceted evaluation of integrated HER functionality, interoperability and security. In addition, certified products meet or exceed applicable proposed federal standards—those that exist on the date of certification—for certified EHR technology to support the 2011-2012 incentives under ARRA. Proposed federal standards were updated on December 30, 2009, after the inspection of Compulink's Advantage/EHR. As part of the inspection, key aspects of successful use are verified at live sites, and product usability is rated. For more information, visit compulinkadvantage.com.
CCHIT is an independent, 501(c)3 nonprofit organization recognized by the Department of Health and Human Services since 2006 as an official certification body for electronic health records. Information on CCHIT, Certified products and Preliminary ARRA certified technology is available at cchit.org and ehr decisions.com.
Website Touts Insurance Designed Exclusively for Physicians
PhysicianInsure, providing physicians and their families and business partners with financial protection since 1992, has a new website, PhysicianInsure.com, specializing in quick and efficient quote requests.
The website allows physicians to determine the amount of insurance needed; offers an educational component through a comprehensive FAQs section, statistics, and product guides; and provides the names of trusted, recommended fee-only financial advisors in the physician's area upon request. PhysicianInsure also offers a speakers bureau and articles for trade publications, reinforcing the importance of insurance for physicians to protect their practice, their family life, and their legacy.
The types of insurance offered include disability insurance, term life insurance, universal life insurance, long-term care insurance and annuities. The website contains sections devoted to non-traditional families and confidential policy review services to help determine if existing coverage is as adequate now as it was when the policy was purchased.
For information, visit the website or call (877) 962-8737.
Anterior Chamber Open-Angle Eye Model from Gulden
The new Anterior Chamber Open-Angle Eye Model from Gulden Ophthalmics helps the eye-care professional explain and demonstrate glaucoma, its treatment and the surgical options available.
The model enables the user to explain the anterior chamber components of the eye, indicting the iris, cornea, Schlemm's canal, the ciliary body and the trabecular meshwork.
Patients can visualize the angle formed by the junction of the iris and cornea at the periphery of the anterior chamber in three dimensions, helping them to better understand open-angle and angle-closure glaucoma. The eye model also helps patients to understand how their intraocular pressure is determined, which may be an incentive for adherence to their eye drop regimen. The professional can demonstrate how the causes and role of elevated IOP.

In addition to standard eye models available from stock, Gulden can design and produce custom eye models that help eye-care professionals save time and increase patient understanding.
For information, call (215) 884-8105, fax (215) 884-0418, email: info@guldenophthalmics.com, or visit guldenophthalmics.com.
Pearl from the Deep
Paul M. Karpecki, OD, FAAO
Diagnosing Significant Ocular Surface Disease Prior to Surgery
There are many reported complications involving the cornea following cataract and refractive surgery, including chronic staining, exposure keratopathy and corneal thinning, persistent epithelial defects and even corneal melts. It is important to manage all ocular surface disease such as dry eye and lid disease prior to surgery to help with quality of vision and accurate biometry measurements, but it is the advanced OSD patient, such as a Sjögren's patient or patient with rosacea or rheumatoid arthritis, who can lead to a difficult postoperative course. Identifying and treating these patients prior to surgery is imperative to an uneventful post-surgical recovery.
A key pearl to managing any ocular surface disease secondary to a systemic cause is to manage the systemic disease. In addition to a thorough ocular examination, every potential surgical patient evaluation should also include observing the following four areas:
1. The skin around the face for signs of rosacea and then potential ocular rosacea.
2. The eyelids for lid laxity, ectropion and any significant blepharitis.
3. Cranial nerve function—rule out a Bell's palsy or a previous stroke affecting one side of the face that results in incomplete lid closure and the risk of corneal exposure or corneal thinning issues.
4. The patient's hands—to rule out rheumatoid arthritis and even a case of secondary Sjögren's syndrome.
Women most often manifest signs of rosacea on the cheeks and men on the nose and forehead. Patients should be treated prior to surgery with a course of doxycycline to address any potential ocular rosacea.
Patients with significant lid laxity or ectropion should consider surgical repair before cataract surgery. Significant blepharitis should be treated with antibiotics, hot compresses, lid hygiene, etc.
Patients with exposure keratopathy from a Bell's palsy or previous stroke should be examined closely for any inferior corneal thinning and also receive prior treatment with ample lubrication and management of the ocular surface.
Patients with arthritis must ensure that their disease is under control prior to surgery. It is very difficult to slow a rheumatoid corneal melt without significant systemic medications and better to have these in place prior to surgery. If the patient also experiences severe xerostomia, then Sjögren's syndrome should be considered and also be managed prior to surgery. Evoxac 30 mg t.i.d. has shown wonderful success in these patients and should be considered starting about a month prior to surgery.
Following this guide of four key areas to look at beyond the slit lamp and fundus examination will allow your cataract patients to experience greater results in the postop course and prevent some potential serious complications.



