A U.S.-European research group reports that increases in annual cost for glaucoma management are related to higher baseline intraocular pressure, higher baseline stage, medication and surgery.
The retrospective study included 151
Among 149 
After adjusting for covariates, patients at higher baseline stages incurred greater costs of glaucoma care in the
The researchers emphasize an accurate cost of blindness needs to be established for future studies involving health-care and economics. Nevertheless, significant potential savings and reductions in annual health-care burden are possible if patients are diagnosed and treated at earlier stages of glaucoma.
(J Glaucoma 2007;16:471-478)
Lee PP, Kelly SP, Mills RP, Traverso CE, Walt JG, Doyle JJ, Katz LM, Siegartel LR.
Eyes Shut Stabilizes Schirmer Results
Administering the Schirmer test with the patient's eyes closed (STc) produces less variable results and more repeatability than with eyes open (STo) in normal subjects, according to researchers in Turkey.
Inclusion criteria were normal ophthalmologic findings except for refractive errors, a Schirmer test (without anesthetic, five minutes, open eye) score of >10 mm, no use of contact lenses, no previous medical or ophthalmic history and no current use of medications. The ST without anesthesia was applied three times with the eyes open and three times with the eyes closed for a total of six times with one-day intervals. To minimize an order effect of the two tests, the participants successively underwent STo and STc in an alternating order. The order was also alternately changed for each patient. The intraclass correlation coefficients (ICCs) and their 95 percent confidence intervals (CIs) were calculated to assess test-retest reliability of the STo and STc.
Twenty-eight eyes of 14 participants (11 women, three men, age range 21 to 44) were included. STc scores were found to be lower than the STo scores in general, and the difference between the two test results was statistically significant (right eyes: t=2.033, p=0.048; left eyes: t=3.474, p=0.004). The ICC was 0.632 (right eyes) and 0.618 (left eyes) for STo and 0.943 (right eyes) and 0.933 (left eyes) for STc.
The researchers admit to limitations with the study. The study was conducted on patients that did not have dry eye, with baseline ST scores of >10 mm. A higher reliability of STc than STo in patients with dry eye cannot be extrapolated from the results because of reduced corneal sensitivity in reported dry-eye patient populations. Another limitation is any possible influence of factors like test anxiety, test familiarity and order effect of the administrations on the results. The small number of participants is another. They call for further studies comparing repeatability of STo and STc in patients with dry eye to extend the results to dry-eye populations.
(Cornea 2007;26:903-906)
Serin D, Karslioglu S, Kiyan A, Alagöz G.
Stable Posterior Cornea Post-LASIK
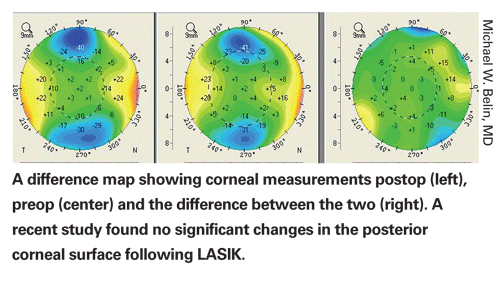
In a study population, no patient had significant forward protrusion of the posterior corneal surface a mean of 14 months after laser in situ keratomileusis, according to researchers in
The Pentacam was used to image the corneas in 52 consecutive patients presenting for their one-year post-LASIK follow-up. One hundred two post-LASIK myopic eyes were prospectively evaluated. Only post-LASIK myopic eyes were included because most reports of posterior corneal surface are of changes after LASIK in these individuals. There were no exclusion criteria.
Changes in the central posterior surface were determined by subtracting the postoperative elevation data from the preoperative elevation data based on the maximal difference in the central 4-mm zone. The reference best-fit sphere was determined by the central 9-mm zone of the postop cornea. The difference in elevation was determined to be the displacement of the posterior corneal surface.
The postop imaging took place a mean of 13.6 months (range, nine to 19 months) after LASIK. The ratio of right to left eyes was equal. The mean age of the 31 men and 21 women was 37.7 ±9.6 years (range 22 to 56 years). Two patients had LASIK in one eye only. The mean spherical equivalent correction was -4.33 ±1.87 D (range -0.75 to -10 D).
Posterior corneal surface measurements in the 102 eyes were very stable at one year. The mean posterior corneal displacement was -0.47 ±4.8 µm (range +7 to -10 µm). No post-LASIK eye had significant posterior corneal ectatic changes. Two eyes had forward posterior corneal displacement of 10 µm, the greatest change in the study population.
The researchers state that previous studies in which subclinical ectasia commonly occurred in normal post-LASIK corneas may have been erroneous secondary to limitations in measuring the posterior surface and a failure to maintain a common reference surface to compare measurements. Contrary to results in previous studies, this study found progressive changes to the posterior corneal surface to not routinely occur after LASIK performed within established parameters.
(J Cataract Refract Surg 2007;33:1366-1370)
Ciolino JB, Khachikian SS, Cortese MJ, Belin MW.