THE CENTER FOR MEDICARE & MEDICAID SERVICES last month announced a major change in its policy regarding reimbursement for cataract surgery. Announced by Mark B. McClellan, MD, PhD, the ruling clarified the agency's payment rules to present beneficiaries with the choice to receive presbyopia-correcting intraocular lenses. Prior to this ruling, limitations on Medicare payment prevented beneficiaries from receiving these lenses. Now beneficiaries who choose to purchase this additional feature will be able to do so.
The agency specifically excludes correction of common refractive errors, such as presbyopia, from Medicare coverage. This ruling clarified that a beneficiary may request insertion of a presbyopia-correcting IOL in place of a conventional IOL following cataract surgery.
With respect to facility charges, CMS states that "the beneficiary is responsible for payment of that portion of the facility charge that exceeds the facility charge for insertion of a conventional IOL following cataract surgery. In addition, the beneficiary is responsible for the payment of facility charges for resources required for fitting and vision acuity testing of a presbyopia-correcting IOL that exceeds the facility charges for resources furnished for a conventional IOL following cataract."
With respect to physician services, CMS states that "the beneficiary is responsible for payment of physician services attributable to the non-covered functionality of a presbyopia-correcting IOL inserted following cataract surgery. In determining the physician service charge, the physician may take into account the additional physician work and resources required for insertion, fitting, and vision acuity testing of the presbyopia-correcting IOL compared to insertion of a conventional IOL. The beneficiary is responsible for payment of the charges for physician services that exceeds the physician charge for insertion of a conventional IOL following cataract surgery."
For more information, visit the CMS website at http://www.cms.hhs.gov/rulins/.
Some Surprises in Annual ASCRS Surgeon Survey
AT THIS YEAR"S MEETING OF THE AMERICAN SOCIETY of Cataract and Refractive Surgery, ophthalmologists David Leaming, MD, of Palm Springs, Calif., and Richard Duffey, MD, of Mobile, Ala., shared the results of their 2004 survey of cataract and refractive surgeons. The survey was mailed to 6,300 ophthalmologists, and around 12 percent of them (773 surgeons) responded.
Dr. Duffey, who manages the refractive surgery section of the questionnaire, finds several of the responses interesting.
"I was a little surprised that Orbscan use isn't continuing to grow," he says. "It's kind of leveled off at 18 percent over the last six years, and has been pretty flat over the last two to three years. I really thought that standard-of-care issues were going to force surgeons to make sure that they were looking at posterior floats. Even though some people don't believe it, the posterior float does give you a bit of extra information that might keep you from doing surgery that you might have otherwise done."
Dr. Duffey also finds the number of surgeons who actually get refractive surgery performed on themselves interesting.
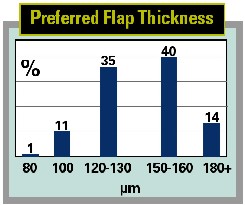
Another intriguing finding is that less than a quarter of the LASIK surgeons measure true flap thickness in the OR.
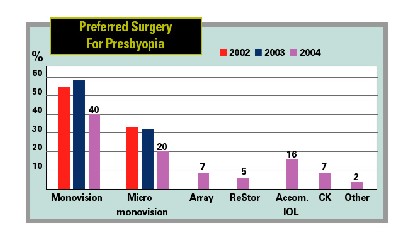
by the survey's being last fall when attitudes may have been different than they are now, monovision LASIK was still the preferred surgery for presbyopes: 40 percent of surgeons chose it.
"What that's telling you is we did not yet have a great, truly presbyopic procedure in the 2004 survey," says Dr. Duffey. "The Crystalens is starting to make some headway. And now the ReStor will start to make even more, just because so many people are used to acrylic-platform IOLs."
Some other refractive findings:
• Seventy-seven percent of respondents say 250 µm is adequate for the residual stromal bed in LASIK.
• Fifty-eight percent of surgeons say they're using mitomycin-c for their refractive procedures.
• Only 35 percent will do refractive surgery on a one-eyed patient.
• Sixteen percent of surgeons are using accommodative IOLs.
• Intralase users are up to 9 percent from 3 percent in 2003's survey.
• Fully three-quarters of the respondents now do wavefront-guided procedures, compared to just over 50 percent in the last survey. They charge an average of $300 per eye more for them compared to conventional.
On the cataract surgery side, some findings included:
• In terms of anesthesia, the numbers haven't changed much from last year's survey, with 62 percent using some form of topical (vs. 61 percent last year), 17 percent using retrobulbar with or without a facial block (vs. 21 percent last year) and 19 percent using peribulbar anesthesia (compared to 17 percent last year). Around 2 percent use blunt sub-Tenon's injection.
• Seventy-three percent of the surgeons make their incisions in the clear or near-clear cornea, up a little from last year's 70-percent mark. Fifteen percent use the limbus and 12 percent use the sclera.
• The IOLMaster has garnered more support, with 35 percent saying they use it for preop biometry, compared to 28 percent in 2003's survey. Applanation A-scan decreased to 49 percent from 61 percent last year. Surgeons saying they use immersion A-scan increased to 16 percent, compared to 12 percent last year.
• The fourth-generation fluoroquinolones continue to dominate the realm of antibiotics: Forty-six percent use Zymar (vs. 33 percent last year) and 43 percent use Vigamox (compared to just 28 percent in the 2003 survey). As for the rest of the respondents, 3 percent use tobramycin, 2 percent use ciprofloxacin, 2 percent use levofloxacin and 2 percent use ofloxacin.
• Interestingly, though 17 percent said they use micro phaco last year, only 3 percent say the same in this year's survey.
At May's ARVO meeting, Glenn J. Jaffe, MD, professor of ophthalmology, a vitreoretinal specialist and director of the Uveitis Service at Duke University Eye Center, presented the results of the analysis of two-year data from the Phase IIb/III randomized, dose-masked, multicenter clinical trial of Bausch & Lomb's Retisert (fluocinolone acetonide intravitreal implant) for the treatment of chronic noninfectious uveitis affecting the posterior segment.
In the trial, conducted at 26 centers in the United States and at one center in Singapore, 278 patients with noninfectious uveitis of the posterior segment were randomized to receive either a 0.59-mg or a 2.1-mg implant in the affected eye, or in bilateral cases, in the more severely afflicted eye. The fellow eye was implant-free.
|
|
|
The Retisert implant. |
Dr. Jaffe presented the aggregate results of the two formulations. At two years, there was a statistically significant lower rate of disease recurrence (p<0.0001) in those eyes with Retisert (11.2 percent) compared to the recurrence rate in the year prior to enrollment (59.7 percent) and compared to the rate in the fellow eyes (50 percent) after two years. There were also statistically significant decreases in systemic use of immunomodulatory therapy and in periocular corticosteroid injections post implantation (p=<0.0001).
The use of systemic steroid/immunosuppressive therapy declined from 52.5 percent of patients at enrollment to 12.5 percent two years post-implantation. Similarly, the use of periocular injections of steroids declined from 68 percent of patients in the year prior to enrollment to 9.7 percent in the two years post implantation. In the fellow eyes, the use of the injections increased from 30.4 percent in the year prior to enrollment to 45.3 percent through two years, a statistically significant increase (p=<0.0001).
The use of topical steroid drops declined in the eyes with Retisert, from 35.7 percent at enrollment to 27.8 percent at two years (p=0.05), while the use of topical steroid drops increased in the fellow eyes from 25.3 percent at enrollment to 35.6 percent (p=0.0008).
In the eyes with Retisert, there was a statistically significant improvement in visual acuity (p<0.0001) after two years, and 24.3 percent of the study eyes had an improvement in vision of three or more lines, compared to 5.3 percent of the fellow eyes.
The most common adverse events included cataract progression and increased intraocular pressure, which were anticipated. These were well-managed by conventional means including cataract surgery and the use of eye drops or filtering surgery.
At two years, in the phakic eyes with Retisert, 89.4 percent had undergone cataract extraction compared to 13.3 percent of the fellow eyes.
Eye drops for the control of elevated IOP were being used by 53.7 percent of the study eyes and 20.2 percent of the fellow eyes, compared to 14 percent of the study eyes and 10.9 percent of the fellow eyes at enrollment. Also, 30.6 percent of the implanted eyes required filtering surgery to control elevated IOP compared to 0.4 percent of the fellow eyes.
Patients in the study will be followed for an additional year.
FDA Priority Review For Alcon's Nevanac
THE U.S. FOOD AND DRUG ADMINISTRATION ACCEPTED Alcon's new drug application for Nevanac (nepafenac 0.1% ophthalmic suspension) for the treatment of pain and inflammation associated with cataract surgery and has granted the application a priority review.
Phase-III clinical data presented at April's ASCRS meeting demonstrated that Nevanac suspension dosed three times per day, in the absence of steroid therapy, was effective in controlling pain and postoperative inflammation associated with cataract surgery. More than 80 percent of patients treated with Nevanac suspension were pain-free on day one, compared to only 40 to 50 percent in the placebo group. By day 14 approximately 95 percent of patients were pain-free when treated with the drug, compared to 45 to 60 percent of patients in the placebo group. Results for inflammation control showed that more than 85 percent of patients treated with Nevanac suspension had no clinically significant inflammation at day 14, compared to approximately 47 percent of patients in the placebo group. These efficacy measurements of inflammation and pain were statistically significant. Nevanac suspension was safe and well-tolerated in both clinical studies, Alcon reports.
In total, the two clinical trials involved 30 investigators who evaluated efficacy in 688 patients. Both trials were randomized, double-masked, placebo-controlled, parallel group studies. Drug or placebo administration commenced one day preoperatively and continued for 14 days after surgery. Patients were evaluated at baseline and at surgery, then at one, three, seven and 14 days after surgery. One study had a dosing regimen of three times per day, while the other included dosing once, twice or three times per day.
Nevanac suspension is a prodrug that penetrates ocular tissues and is converted intraocularly into amfenac, a potent nonsteroidal anti-inflammatory drug. Because of its prodrug structure, Nevanac suspension penetrates the cornea rapidly, reaching target sites while minimizing surface accumulation and potentially reducing the risk of ocular surface complications, Alcon reports.
CDC: Children Don't Receive Adequate Eye Care
A RECENT CENTERS FOR DISEASE CONTROL AND PREVENTION report shows that roughly one in three children in America received eye-care services before their sixth birthday. The "Visual Impairment and Use of Eye-Care Services and Protective Eyewear Among Children" findings, published in the Morbidity and Mortality Weekly Report, were based on a national survey with more than 12,000 participants.
Asian and Hispanic children were the least likely to get their vision checked, compared to black or white children. This is especially concerning since the report also showed that Hispanic children had a higher prevalence of visual impairment and blindness than white children (3.6 percent and 2.3 percent, respectively).
TTT4CNV Trial Reports Positive Results
IRIDEX CORP. ANNOUNCED LAST MONTH THAT additional follow-up data confirmed a significant clinical benefit in a group of patients with wet age-related macular degeneration who were treated with the transpupillary thermotherapy laser protocol when compared to the sham-treated (placebo) control group in the TTT4CNV clinical trial.
Elias Reichel, MD, chairman of the trial and associate professor of ophthalmology at the New England Eye Center, Tufts University School of Medicine, presented the results covering 305 participating patients at May's ARVO meeting.
The updated results confirmed and expanded on the initial results reported in February 2005 that a subgroup of patients, who were enrolled into the study with baseline visual acuity of 20/100 or worse, benefited from TTT treatment. Within the TTT4CNV trial, about 41 percent of the patients enrolled had baseline vision of 20/100 or worse. Specifically, at 12 months following treatment 23 percent of TTT treated eyes in this subgroup improved vision by one or more lines and 14 percent of TTT treated eyes improved vision by three or more lines compared with none of the eyes in the placebo treated control group. Further, at 18 months, there was a two-line benefit in preserving vision in this subgroup when compared to placebo-treated eyes. Specifically, TTT treated eyes on average lost two lines of visual acuity while placebo-treated eyes lost four lines. These findings were statistically significant.