AREDS Supplementation
The AREDS study suggested that supplementing the diet of patients with early or intermediate AMD with vitamin C, vitamin E, beta-carotene and zinc could significantly reduce the risk of progressing to advanced AMD.2 The AREDS 2 study indicated that replacing the beta carotene in the original AREDS formula with lutein and zeaxanthin may offer similar protection against developing advanced AMD.3 The AREDS2 formulation may be safer for smokers and former smokers, as taking beta-carotene is associated with an increased risk of lung cancer in these patients. Commercial supplements such as PreserVision AREDS and PreserVision AREDS 2 (Bausch+ Lomb) are routinely recommended to patients who are at risk of progressing to advanced AMD, at least in part because there’s currently nothing else to offer patients to avoid geographic atrophy.
“Even practitioners may sometimes be a little hazy with regards to what they tell their patients about the effects of the vitamins,” acknowledges Wai T. Wong, MD, PhD, clinician-scientist, Unit on Neuron-Glia Interactions in Retinal Disease, National Eye Institute. “AREDS supplementation is given to patients with intermediate AMD who have large drusen in their maculas. The question remains, what exactly does it do for them? If you look very carefully at the trial data, what it actually shows is that AREDS supplements can reduce the risk of progressing from intermediate to wet AMD. It can reduce that rate by about one-quarter or one-third, depending upon the AREDS or AREDS2 formulation. But if you look at that data, it actually doesn’t reduce the risk of progression from intermediate to atrophic AMD at all, just the risk of progression to wet AMD,” he says. “Among patients who already have atrophic AMD and whose GA lesions are increasing in size, again, the data do not show that AREDS supplementation is helpful in slowing down the growth in GA areas.”
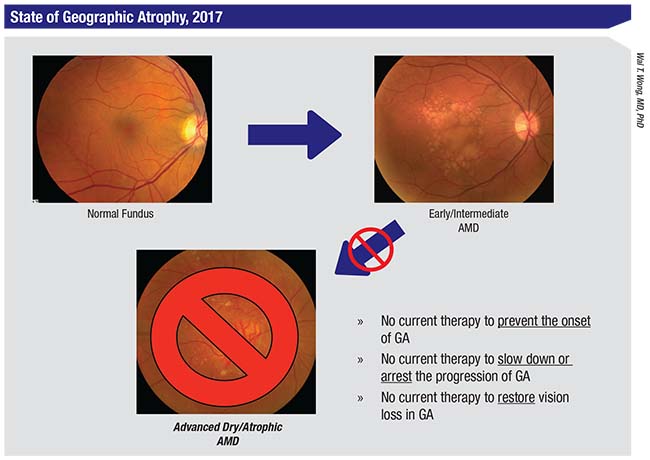 |
| This slide summarizes the current state of affairs regarding therapeutic options to prevent, treat, or repair cellular damage from geographic atrophy, the advanced stage of nonexudative or “dry” AMD. Fundus photos show a normal eye (top left) progressing to early/intermediate AMD with drusen (top right), to advanced AMD with geographic atrophy (bottom center). |
Even so, Dr. Wong continues to recommend AREDS supplementation for all patients at risk for developing wet AMD in the hope of sparing GA patients the “double whammy” of conversion from GA to wet AMD. “There’s some theoretical evidence that it might be the case that if you have advanced atrophic AMD, the vitamins might decrease the risk of getting wet AMD,” he explains. “However, that’s not been directly tested in a large-enough study. Therefore, the main contribution of AREDS supplements is to reduce the risk of getting from intermediate AMD to wet AMD. It’s still worth doing, however.”
Lampalizumab
Dysregulation of the complement system—part of the body’s defense system against invading pathogens—has long been implicated in RPE damage and photoreceptor cell death in AMD. Complement factors have been found at elevated levels in the bloodstreams of AMD patients4 as well as in drusen.5 Once activated, the complement cascade sets off a series of reactions that results in inflammation and cell death. Different complement proteins have been drug targets in the quest to combat GA, with no clear winners so far.
Lampalizumab (Genentech) is a monoclonal antibody fragment designed to alter the activation of complement factor D in order to throw a wrench into the workings of an inflammatory cascade. Dr. Wong likens the regulation of the complement factor cascade to a car with multiple gas pedals and brakes. “What lampalizumab is trying to do is bind up complement factor D, which is actually one of the positive regulators—one of the gas pedals that would drive forward towards complement activation,” he explains.
In a Phase II study (MAHALO)6 patients with geographic atrophy who received monthly injections saw a 20.4-percent reduction in the rate of growth of their areas of GA at 18 months; a subgroup of patients who tested positive for a biomarker for complement factor I (CFI) experienced even greater reduction (44 percent) in the rate of change in their GA lesions.
“The study investigators didn’t exclusively recruit subjects with a genotype thought to respond to the drug,” notes Dr. Wong, who adds that these findings emerged in a post hoc analysis. “The Phase II results are in some sense promising, but inconclusive, because it’s a relatively small study and the overall effect size is actually quite small. Only on post hoc analysis did they find that this treatment was more effective in a subset of patients with a certain complement gene makeup. So you’re left wondering if this could have happened by chance. That is one question raised by the Phase II study,” he says.
The Phase III trials, CHROMA (identifier: NCT02247479) and SPECTRI (identifier: NCT02247531), prospectively genotyped patients to see whether the CFI patients would be strong responders to lampalizumab in a larger sample. The CHROMA and SPECTRI trials are identically designed, multicenter, interventional studies enrolling 936 patients apiece, with 60 percent of the study arm positive for the CFI biomarker.
Genentech released the early results of SPECTRI7 in September 2017: Lampalizumab given to the treatment arm once monthly or every six weeks failed to meet the study’s primary endpoint, which was reduction in change in GA growth rate at 48 weeks. SPECTRI patients are no longer being dosed with lampalizumab, pending results from CHROMA, which are expected sometime in November 2017.
APL-2
In August 2017, Apellis Pharmaceuticals (Louisville, Ky.) announced the results of a Phase II study8 of its own complement-factor inhibitor, APL-2, a drug also being investigated to treat a blood disorder called paroxysmal nocturnal hemoglobinuria. APL-2 is a complement factor 3 (C3) inhibitor.
The FILLY trial, a multicenter, randomized, single-masked, sham-controlled study, involved 246 patients. Study eyes received intravitreal injections of APL-2 either monthly or semimonthly for 12 months, and were monitored for six additional months. The primary endpoint was reduction in GA growth versus sham eyes from baseline to month 12 as measured by fundus autofluourescence photography. Compared to the sham eyes, the study eyes getting monthly APL-2 experienced a 29-percent reduction in the rate of GA lesion growth; the semimonthly APL-2 eyes showed a 20-percent reduction compared with sham eyes. Adverse events included an increased risk of onset of neovascular AMD, particularly in patients with wet AMD in the fellow eye. This was treated with standard anti-VEGF therapies.
During the subsequent six months of the FILLY trial, a post hoc analysis showed further reductions in the growth rate of GA in the monthly and semimonthly eyes: 47 percent and 33 percent, respectively. Apellis plans to conduct a Phase III trial in the near future.
Brimonidine
Brimonidine (Alphagan; Allergan) is a longstanding IOP-lowering therapy for glaucoma. Studies have suggested that topical brimonidine has a neuropotective effect in glaucoma, with less visual-field progression in treated eyes. This prompted Allergan to explore whether the drug is a viable treatment for the reduction of GA progression, according to William R. Freeman, MD, distinguished professor, vice chair and director of the Jacobs Retina Center at University of California, San Diego.
Brimo DDS, an intravitreal brimonidine delivery system based on the manufacturer’s Ozurdex (dexamethasone) implant for DME and uveitis, is currently in trials for GA. Brimonidine, an alpha-2 adrenergic agonist, may have a protective effect on the retina by inducing the release of neurotrophic substances that protect the RPE and photoreceptor cells. The Brimo DDS implant allows a sufficient amount of brimonidine to reach the retina for possible therapeutic benefit, and may reduce treatment burden compared to intravitreal injections of free drug.
The Brimo DDS device currently being studied is a 25-gauge implant that researchers hope will have neuroprotective properties that will spare cells and preserve vision in dry AMD. “You could say that brimonidine is being repurposed, but it’s never really been used for a retinal disease,” says Dr. Freeman. “It appears to be a neurocytoprotectant, and I use that term because the retinal pigment epithelial cells, strictly speaking, are not neural cells, but brimonidine does seem to protect those. So the decision was made to try it in geographic atrophy.”
Dr. Freeman presented the findings of the Phase IIa study of Brimo DDS Generation 1 (22-gauge) implant at last year’s American Academy of Ophthalmology meeting in Chicago. “The Phase IIa study showed some evidence of efficacy,” he says. “It is not published, but it shows a reduction in the rate of atrophy—the area of dead cells. Increases in that area are lower with brimonidine that’s been delivered with this system. Depending on how you do the statistics, after 12 months, the reduction was statistically significant compared to sham.
“In the Phase IIb study, they’re using an improved device,” Dr. Freeman continues. In
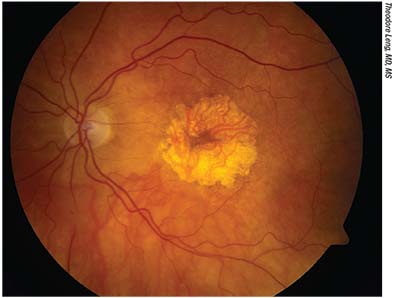 |
| Color fundus photograph of an eye with central geographic atrophy. Proposed treatments for dry AMD have sought to regress drusen without giving rise to GA lesions, reduce the rate of GA growth, or to replace macular cells lost to GA. |
The BEACON study will test the new-generation Brimo DDS implant’s safety and efficacy. The trial is ongoing but no longer recruiting. It will look at 311 patients in a multicenter, double-masked study to compare changes in GA lesions and BCVA in eyes treated with monocular 400-μg brimonidine implants replaced at three-month intervals through month 21 with patients whose eyes are given sham treatment with a needleless applicator (no implant) on the same schedule through month 21. The primary endpoint is change from baseline in atrophic lesion area in the eyes studied, to month 24.
“The lesion size is measured photographically and by autofluourescence,” Dr. Freeman, a BEACON investigator, explains. “Vision is in there, but not as the primary endpoint. You could be losing a big chunk of your vision that doesn’t hit the center, so it’s the area of absent cells. That progresses and you can see it over time, so at every visit photographs are taken.”
While Dr. Freeman is unsure if or when Allergan will release interim data, he estimates that they’ll have two-year data available in late 2018.
Atorvastatin
AMD and atherosclerosis share some risk factors, such as age, smoking and hypercholesterolemia.9 There is some evidence that cholesterol-lowering drugs may decrease the risk of developing AMD;10 however, it has not been demonstrated that statins can retard disease progression.11 A recent pilot study led by Harvard researchers treated patients with large drusen with high-dose oral atorvastatin to investigate if the treatment reduced these drusen.12
“This has not been proposed for treating people with GA,” notes Dr. Wong, “but instead for people with intermediate AMD who are in danger of developing GA.”
The small Phase II study of 26 AMD patients aged 55 and up investigated high-dose (80 mg/day) oral atorvastatin’s ability to reduce drusen volume and improve visual acuity. Of the 23 patients who completed a 12-month minimum follow-up, 10 showed regression of drusen volume as measured by fundus photography and OCT, associated with a gain of 3.3 EDTRS letters over baseline (p=0.06). None of the cohort progressed to wet AMD. The study is small, and per the researchers, fails to take into account the genetic heterogeneity of dry AMD. The authors suggest that statins may be beneficial for some patients with large soft drusen, which are markers of high risk for progression to wet AMD. The patients who didn’t respond to the atorvastatin regimen lost an average of 2.3 letters.
Further study would be needed to see if atorvastatin is a viable path to halting AMD from progressing to its vision-robbing later stages. “A study directly investigating the ability of atorvastatin to prevent GA onset has not been performed,” notes Dr. Wong. “Prevention trials are usually very large and very expensive, and it is helpful to identify a very high-risk population so that the event rate of GA onset is sufficiently high to as to reveal a significant treatment effect. That’s the challenge of developing outcome measures. A specific GA-prevention trial has never been done before, so the design implications are going to be novel.
“There’s also a little debate about drusen,” he continues. “Having lots of drusen generally means more risk, but GA actually forms from an acute collapse of all these drusen. Drusen build up, collapse, and the onset of GA ensues. So the disappearance of drusen is sometimes a bad sign. But what the investigators from Harvard think is that maybe the drug will cause the drusen to go away without the negative consequences, reducing the risk of GA onset. Part of the interest in cholesterol is that, in addition to complement genes appearing important to the control of AMD risk, lipid genes have also been found to have an important effect on controlling risk. We don’t really know why, or what the mechanisms are, but looking at things like Lipitor is one way of broaching the question.”
Photobiomodulation
Laser therapy as a proposed method of controlling drusen and heading off the visual complications of advanced AMD has not yet yielded clear or long-term benefits.13 Although verteporfin photodynamic therapy was the standard of care for neovascularization before the advent of anti-VEGF therapy, there has been no counterpart for patients with dry AMD.
Graham F. Merry, MBBS, LMCC, and colleagues have been working with LED-sourced light treatments to regress drusen in patients diagnosed with dry AMD, and to correspondingly improve their visual function with these photobiomodulation treatments. The researchers believe that photobiomodulation therapy has anti-inflammatory properties and antioxidant properties that encourage phagocytosis in RPE cells, and can regress drusen without contributing to GA. Their recent study14 looking at 42 eyes with dry AMD treated with three weekly sessions of photobiomodulation therapy for three weeks showed improvements in mean BCVA and contrast sensitivity, together with decreases in drusen volume and central thickness as measured via OCT and FAF.
“Blue Sky Right Now”
“If you want to break down all the GA trials, you can break them out into the prevention of progression to GA; trying to slow down the growth of GA; and, finally, bringing back whatever has been lost in GA,” says Dr. Wong. “It’s completely blue sky right now: We don’t really have a lot in terms of established mechanisms to guide us.”
Oxidative stress has long been implicated in AMD. Cumulative oxidative stress may drive progressive retinal damage, and treatments that inhibit the production or effect of reactive oxygen species (ROS) are another investigative avenue. “Clearly, people who smoke, for instance, have an increased risk of advanced AMD, and that’s well established,” says Dr. Wong. “In 2010, we at the NEI had investigated an experimental compound administered as an eye-drop that acts as a free-radical scavenger in order to reduce oxidative stress.15 That’s one of the things investigators may continue to pursue. But so far, there’s nothing that’s demonstrated efficacy with respect to GA.”
One therapeutic avenue he and his colleagues are actively investigating is the anti-inflammatory effect of a broad-spectrum tetracycline drug. “We’re currently doing an oral minocycline study,” says Dr. Wong. “In addition to acting as an antibiotic, it also has anti-inflammatory properties and it penetrates into the retina pretty well. We’re doing the minocycline study in collaboration with two study sites in the U.K. There is also a concurrent study at the University of Virginia that is using another tetracycline medication. We’re using minocycline; they’re using doxycycline. The strategies are quite similar: to suppress the immune cells within the eye to see if that is helpful in slowing the growth of GA.”
Stem-cell therapies represent a line of investigation that may one day lead to a way of replacing photoreceptor or retinal pigment epithelial (RPE) cells that have been destroyed by GA, rather than preventing or slowing GA. The proposed methods vary, according to Dr. Wong. “Different people have different ideas about the best way to replace the cells. Some people just inject embryonic stem cells or cells from other donor sources at various stages of differentiation, and let them float free until they settle down, where hopefully they’ll take root, rather like sprinkling grass seed on a bare patch of lawn. Others differentiate the cells by growing them on a membranous scaffold so they’re lined up like a sod patch that you can place exactly where they need to be. The plan is that the scaffold will dissolve over time, leaving behind a single layer of surviving RPE cells. These are the approaches that are being tried or planned,” he reports.
He believes one thing is certain, however. “A lot of groups are trying different things, and success in any of these trials—even a small amount—will redirect the field. We’ve been barking up different trees, and the first one that bears fruit is going to re-orient the field in that direction.”
Dr. Wong is a clinician-scientist with the NIH, and has no interests to disclose. Dr. Freeman has previously served as a consultant to Allergan, but is not personally remunerated as an investigator in the brimonidine trial described in this article.
1. Klein R1, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: The Beaver Dam Eye Study. Ophthalmology 2007;114;2:253-62.
2. AREDS Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with Vitamin C and E, beta carotene, and zinc for AMD and vision loss: AREDS report no. 8. Arch Ophthalmol 2001;119:10:1417-36.
3. Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration:the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013;309:19:2005-15.
4. Scholl HP, Charbel Issa P, Walier M, Janzer S, Pollok-Kopp B, Börncke F, Fritsche LG, Chong NV, Fimmers R, Wienker T, Holz FG, Weber BH, Oppermann M. Systemic complement activation in age-related macular degeneration. PLoS One 2008;3;7:e2593.
5. Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res 2001;20;6:705-32.
6. Regillo CD. Lampalizumab (anti-factor D) in patients with geography atrophy: The MAHALO phase 2 results. Paper presented at: the 2013 Annual Meeting of the American Academy of Ophthalmology; November 16-19, 2013; New Orleans, LA.
7. Genentech press release. September 8, 2017. Genentech provides update on first Lampalizumab phase III study for geographic atrophy, an advanced form of age-related macular degeneration. https://www.gene.com/media/press-releases/14681/2017-09-08/genentech-provides-update-on-first-lampa. Accessed 10/2/17.
8. Apellis Pharmaceuticals press release. August 24, 2017. Apellis Pharmaceuticals announces that APL-2 met its primary endpoint in a Phase 2 study in patients with geographic atrophy, an advanced form of age-related macular degeneration. http://www.apellis.com/pdfs/Press%20Release%20FILLY%2012%20Month%20Results%20FINAL%20FINAL%20170823.pdf. Accessed 10/10/17.
9. Klein RK, Cruikshanks KJ, Nash SD, et al. The prevalence of age-related macular degeneration and associated risk factors: The Beaver Dam Offspring Study. Arch Ophthalmol 2010;128;6:750–8.
10. McGwin Jr G, Xie A, Owsley C. The use of cholesterol-lowering medications and age-related macular degeneration. Ophthalmology 2005;112;3:488-94.
11. Al-Holou SN, Tucker WR, Agrón E, et al. The association of statin use with age-related macular degeneration progression: The Age-Related Eye Disease Study 2 Report Number 9. Ophthalmology 2015;122;12:2490-6.
12. Vavvas DG, Daniels AB, Kapsala ZG, Goldfarb JW, Ganotakis E, et al. Regression of some high-risk features of age-related macular degeneration (AMD) in patients receiving intensive statin treatment. EBiomedicine 2016; 5:198-203.
13. Complications of Age-Related Macular Degeneration Prevention Trial Research Group. Laser treatment in patients with bilateral large drusen. Ophthalmology 2006;113:1974–1986.
14. Merry GF, Munk MR, Dotson RS, Walker MG, Devenyi RG. Photobiomodulation reduces drusen volume and improves visual acuity and contrast sensitivity in dry age-related macular degeneration.
15. Wong WT, Kam W, Cunningham D, et al. Treatment of Geographic Atrophy by the topical administration of OT-551: Results of a Phase II clinical trial. Invest Ophthalmol Vis Sci 2010;51;12:6131-9.



