Pravin U. Dugel, MD
Phoenix
Pseudophakic cystoid macular edema was first described by A. Ray Irvine Jr., MD,1 in 1953 and further elucidated by J. Donald M Gass, MD, in 1969.2 Dr. Gass described that the peak incidence occurred at six weeks postoperatively, and suggested there was a difference between angiographic versus clinically significant PCME, in that only a few patients complained of decreased vision. Clinical PCME is now defined as a Snellen visual acuity of 20/40 or worse. Common symptoms include decreased visual acuity and contrast sensitivity as well as metamorphopsia.
Although the adoption of phacoemulsification over extracapsular cataract removal has decreased the rate of PCME from the 16 to 24 percent range,3 the increasing use of multifocal intraocular lens implants has led to higher expectations from patients. Due to the design of these new implants, which inherently diminish contrast sensitivity, even mild PCME can result in a significant reduction in quality of vision and patient satisfaction. When the Food and Drug Administration approves the use of any new IOL, it states that the "acceptable rate" of clinically significant PCME should be less than 5 percent. Given the rapidly evolving surgical and IOL technology, "clinically significant" PCME may need to be redefined to fit both the patient's visual requirements and expectations.
Incidence
The incidence of PCME following phacoemulsification in uncomplicated, low-risk patients can vary from 2 percent to 12 percent.4,5 The peak incidence is thought to occur six weeks after surgery.2 Factors predictive of the development of PCME in this population include a history of retinal vein occlusion, the presence of an epiretinal membrane, and preoperative prostaglandin use.4 In patients with high-risk characteristics, such as diabetes mellitus, or in complicated cases, the incidence can be much higher. (Heier JS, et al. Characteristics of patients with CME following phacoemulsification. Presented at Annual Meeting of the American
Mechanism
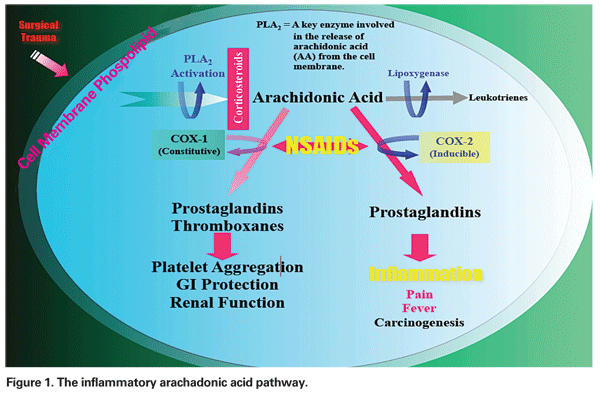
The mechanism by which PCME develops can be multifactorial, having varying degrees of inflammatory and mechanical components. Surgical manipulation of the anterior segment triggers the release of arachadonic acid from cell membranes, which leads to the production of prostaglandins and leukotrienes via the cyclo-oxygenase (COX) and lipoxygenase (LOX) pathways, respectively (See Figure 1). These inflammatory mediators increase vasopermeability, thereby leading to the development of PCME.
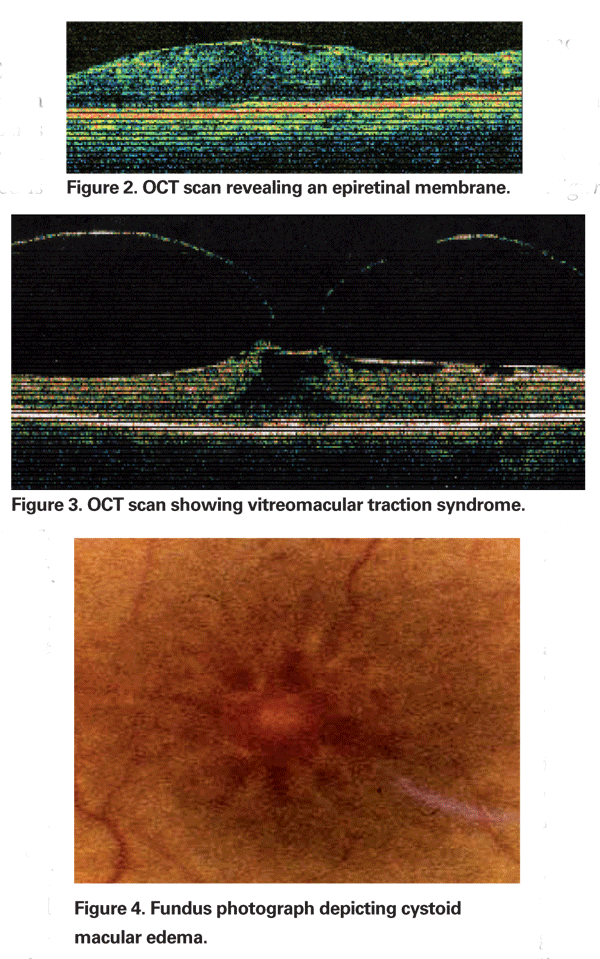
Occasionally, the inflammation produced by cataract extraction can cause contraction of the posterior hyaloid face, leading to distortion of parafoveal capillaries and resultant macular edema. The increasing use of optical coherence tomography has become an important part in the assessment of PCME. OCT images are helpful in identifying a mechanical component of CME, including vitreomacular traction or epiretinal membranes (See Figures 2 & 3).
In rare cases of chronic, refractory PCME in low-risk patients without any other identifiable etiology, the eye must be closely scrutinized for retained lens fragments, IOL malposition or the presence of Propionibacterium acnes infection.
Diagnosis
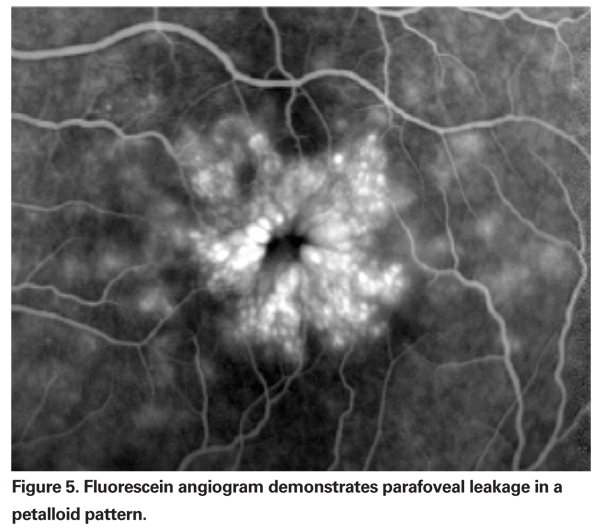
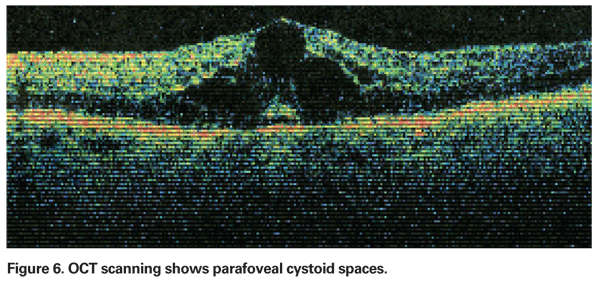
PCME should be suspected when a patient without underlying risk factors complains of decreased vision or metamorphopsia following cataract extraction. Clinically, intra-retinal edema contained in cyst-like spaces in a honeycomb pattern around the fovea can be seen (See Figure 4). On fluorescein angiogram, a petalloid pattern of leakage into the parafoveal intra-retinal spaces can be seen, along with optic disc hyperfluorescence (See Figure 5). OCT can clearly demonstrate these cystoid spaces as well as calculate central macular thickness and total macular volume (See Figure 6). The advantages of OCT are its high sensitivity, non-invasiveness, delivery of anatomic information, and ability to quantify macular edema, although there is a questionable correlation of these values and visual function.
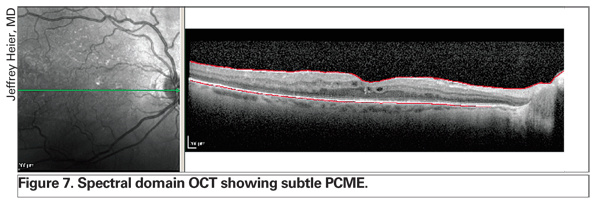
The use of OCT is revolutionizing the management of retinal edema. Newer technology, including spectral domain OCT, delivers better resolution and may detect subtle PCME earlier than slit-lamp biomicroscopy and fluorescein angiography (See Figure 7). In addition, this improved instrumentation allows the clinician to distinguish between exclusively intraretinal edema and subretinal or sub-pigment epithelial fluid, which may provide prognostic information (See Figure 8). It has been the experience of several retinal specialists [personal communication] that the presence of a subfoveal pigment epithelial detachment may portend a worse prognosis.
Prevention
There have been several retrospective reviews and prospective trials evaluating the effect of prophylactic non-steroidal anti-inflammatory drugs on PCME rates when used preoperatively versus postoperatively. In one report, three days of pretreatment with ketorolac tromethamine (Acular) was superior to drops given one hour prior to surgery.6 When compared to treatment with postoperative corticosteroids alone, another group reported a reduction in PCME from 12 percent to 0 percent with two days of preoperative diclofenac sodium (Voltaren) and combination diclofenac/ corticosteroid postoperatively.5
Using OCT to quantify macular volume, researchers found that two days of pretreatment with ketorolac and postoperative combination therapy using ketorolac/steroid led to a 46-percent reduction in macular swelling one month after surgery when compared to a steroid-only control group.7 Recently, a randomized prospective trial comparing three days of preoperative ketorolac and combination therapy to corticosteroid alone reported that, based on OCT, patients in the control group had a 2.4-percent incidence of PCME compared to 0 percent in the combination cohort.8 Although patients in the corticosteroid group had a significantly higher incidence of retinal thickening ≥15 µm, there was no statistical difference in best-corrected visual acuity between the two groups four weeks after surgery.
In a prospective evaluation of three groups assigned to topical indomethacin for three days preoperatively followed by combination therapy, postoperative combination therapy only, or corticosteroid only, the incidence of angiographic PCME was reduced from 32.8 percent (corticosteroid only) to 15 percent (postop combination) to 0 percent (pre- and postop NSAID).9
Another report stated that the addition of nepafenac sodium (Nevanac) to a postoperative corticosteroid regimen reduced PCME rates from 2.1 percent to 0 percent when compared to corticosteroid-only.10 A recent prospective randomized comparison of diclofenac alone versus betamethasone postoperatively found that, at five weeks after surgery, the incidence of angiographic CME was lower in the NSAID group (18.8 percent) than in the corticosteroid group (58.0 percent).11
These studies show that the prophylactic use of NSAIDs reduces the incidence of PCME, and that their efficacy is greatest when started at least three days preoperatively and continued postoperatively for several weeks. However, the exact timing and duration of preoperative and postoperative treatment, and the identification of patients who would benefit most, remain as yet undetermined.
High-risk Characteristics
As mentioned previously, in a series of 1,659 consecutive cases, epiretinal membrane, a history of vein occlusion or preoperative use of prostaglandin analogues greatly increased the risk of PCME development.4 When examining a series of consecutive patients referred to a retinal clinic for management of PCME, another group found high-risk characteristics to be diabetes mellitus, hypertension, previous ocular surgery, epiretinal membrane and complicated cataract extraction. (Heier JS, et al.) Given that a variety of medical and ocular maladies may predispose patients to the development of PCME, identification of these patients and aggressive prophylactic therapy with topical NSAIDs should be utilized to reduce the risk of developing PCME.
Treatments
• NSAIDs. This class of medications inhibits the production of prostaglandins via inhibition of the COX pathway (See Figure 1). As NSAIDs have no effect on preformed or existing prostaglandin levels, treatment before surgical trauma is essential. In a recent review article, the author recommended preoperative treatment with NSAIDs for two days in low-risk patients and one week for high-risk patients, with continuation for at least four weeks postoperatively in both groups.12
Questions still exist as to which NSAID is most effective in preventing or treating PCME.
Newer generation NSAIDs, such as bromfenac sodium (Xibrom) and nepafenac, have modifications to their chemical structure, increasing their ocular penetration and theoretical potency.13 In vitro assays have found that the relative potency of COX-2 inhibition is 18 times greater for bromfenac than ketorolac, although the clinical significance of this data is unknown.14 A study of in vivo pharmacokinetics revealed that aqueous humor concentrations of commercially available nepafenac (nepafenac plus amfenac) is significantly greater than that of either bromfenac or ketorolac.15 However, it is not known whether these differences in penetration translate into improved clinical outcomes. Although numerous marketing claims have been made to imply that the better penetration, structural changes and greater proposed potency of the newer generation NSAIDs translate into increased clinical efficacy, no definitive study has been done to confirm this.
• Combination Therapy. A randomized, double-masked, prospective trial evaluating ketorolac versus prednisolone versus combination therapy for clinically significant PCME found that patients receiving combination therapy reached a mean change of ≥2 lines of vision by their second visit, while the patients in the two monotherapy groups never reached a mean two-line improvement at three month follow-up.16 Patients treated with either combination therapy or ketorolac alone also responded more quickly than patients in the prednisolone group.
• Corticosteroid Injections. Corticosteroids work further upstream in the same pathway as NSAIDs, inhibiting the release of arachadonic acid from cell membrane phospholipids, thereby preventing the formation of both leukotrienes and prostaglandins (See Figure 1).
Debate continues regarding the most efficacious route of administration (sub-Tenon's versus intravitreal), optimal dosage (4 mg versus greater), and patient safety (risks of ocular hypertension, retinal detachment, vitreous hemorrhage and endophthalmitis).
Currently, no randomized clinical trials exist evaluating these modes of treatment, and published results appear contradictory.
One group of investigators found that intravitreal triamcinolone for persistent PCME resulted in improved visual acuity, decreased macular thickness and increased multifocal electroretinogram values.17 Another reported that the use of 4 mg of IVTA in chronic refractory PCME resulted in anatomic and functional improvement after one month from the injection and persisting through at least three months.18 However, the beneficial effect was temporary as visual acuity and macular thickness returned to preinjection levels despite multiple treatments. In still another small series, patients maintained an improvement in acuity and decreased macular thickness following a single dose of 4 mg IVTA for refractory PCME.19
• Acetazolamide. Acetazolamide increases subretinal fluid transport and has been shown to remove foveal cystoid edema in disorders such as retinitis pigmentosa, aphakia and macular epiretinal membrane formation.20-23 There are a handful of case reports correlating the resolution of PCME with use of oral acetazolamide, but to date, no clinical trials have been performed.
• Anti-vascular endothelial growth factor agents. Surgical trauma leads to postop inflammation which can lead to PCME through an increase in the production of vasopermeable factors such as vascular endothelial growth factor. Again, small case series provide contradictory evidence. After intravitreal injection with bevacizumab (Avastin), one group found that 71 percent of patients gained two or more lines of ETDRS vision;24 and another reported that all eyes in their series had a statistically significant improvement in vision.25 However another study reported that intravitreal bevacizumab did not improve visual acuity in patients with PCME.26
• Vitrectomy. Pars plana vitrectomy with membrane peeling may be considered in cases of PCME with a mechanical component as identified either on clinical exam or by OCT, or in cases of chronic refractory edema unresponsive to medical therapy. In a small series, surgical intervention resulted in a greater than three line improvement in best-corrected visual acuity in most patients, and resolution of PCME in all cases.27 There are also case reports in the literature indicating visual improvement following vitrectomy and peeling of the internal limiting membrane.28 As mentioned earlier, pars plana vitrectomy should be considered in cases of retained lens fragments, IOL malposition or P. acnes infection.
Future Directions
As mentioned earlier, corticosteroids work at the beginning of the inflammatory cascade. Posurdex (Allergan) is a biodegradable sustained dexamethasone delivery system that is implanted into the vitreous cavity. Its polymer matrix gradually transforms into lactic and glycolic acid, which are in turn broken down into water and carbon dioxide. In a Phase II study, 54 percent of patients suffering from PCME and uveitis-associated CME showed an improvement of at least 10 letters of acuity at post-implant day 90, as compared to only 14 percent showing a similar result in the observation group. [Allergan Phase II study data, unpublished; personal communication]
Recently, the FDA approved Triesence (Alcon), triamcinolone acetonide, for intravitreal injection during surgery. This drug may be used off-label in the treatment algorithm of recalcitrant PCME.
It is evident that the definition of PCME has been evolving over recent years. With approximately 3 million cataract surgeries performed in the
The authors practice at Retinal Consultants of Arizona,
1.
2. Gass JD, Norton EW. Follow-up study of cystoid macular edema following cataract extraction. Trans Am Acad Ophthalmol Otolaryngol 1969;73(4):665-82.
3. Wright PL, Wilkinson CP, Balyeat HD, et al. Angiographic cystoid macular edema after posterior chamber lens implantation. Arch Ophthalmol 1988;106:740-744.
4. Henderson BA, Kim JY, Ament CS, et al. Clinical pseudophakic cystoid macular edema. Risk factors for development and duration after treatment. J Cataract Refract Surg 2007;33:1550-8.
5. McColgin AZ,
6. Donnenfeld ED, Perry HD, Wittpenn JR, et al. Preoperative ketorolac tromethamine 0.4 percent in phacoemulsification outcomes: Pharmacokinetic-response curve. J Cataract Refract Surg 2006;32:1474-82.
7. Almeida DR, Johnson D, Hollands H, Smallman D, et al. Effect of prophylactic nonsteroidal antiinflammatory drugs on cystoid macular edema assessed using optical coherence tomography quantification of total macular volume after cataract surgery. J Cataract Refract Surg 2008;34:64-9.
8. Wittpenn JR, Silverstein S, Heier J, et al. A Randomized, Masked Comparison of Topical Ketorolac 0.4 percent Plus Steroid vs Steroid Alone in Low-Risk Cataract Surgery Patients. Am J Ophthalmol 2008; 146:554 –560.
9. Yavas GF, Oztürk F, Küsbeci T. Preoperative topical indomethacin to prevent pseudophakic cystoid macular edema. J Cataract Refract Surg 2007;33:804-7.
10. Wolf EJ, Braunstein A, Shih C, et al. Incidence of visually significant pseudophakic macular edema after uneventful phacoemulsification in patients treated with nepafenac. J Cataract Refract Surg 2007;33:1546-1549.
11. Asano S, Miyake K, Ota I, et al. Reducing angiographic cystoid macular edema and blood-aqueous barrier disruption after small-incision phacoemulsification and foldable intraocular lens implantation: Multicenter prospective randomized comparison of topical diclofenac 0.1 percent and betamethasone 0.1 percent. J Cataract Refract Surg 2008;34:57-63.
12. O'Brien TP. Emerging guidelines for use of NSAID therapy to optimized cataract surgery patient care. Curr Med Res & Opin. 2005;21:1131-1137.
13. Walsh DA, Moran HW, Shamblee DA, et al. Antiinflammatory agents 3. Synthesis and pharmacological evaluation of 2-amino-3-benzoylphenylacetic acid and analogues. J Medicinal Chem 1984;27:1379-1388.
14. Waterbury LD, Silliman D, Jolas T. Comparison of cyclooxygenase inhibitory activity and ocular anti-inflammatory effects of ketorolac tromethamine and bromfenac sodium. Curr Med Res Opin 2006;22: 1133-1140.
15. Walters TR, Raizman M, Ernest P, et al. In vivo pharmacokinetics and in vitro pharmacodynamics of nepafenac, amfenac, ketorolac, and bromfenac. J Cataract Refract Surg 2007;33;1539-1545.
16. Heier JS, Topping TM, Baumann W, et al. Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema. Ophthalmology 2000;107:2034-8.
17. Koutsandrea C, Moschos MM, Brouzas D, et al. Intraocular triamcinolone acetonide for pseudophakic cystoid macular edema: Optical coherence tomography and multifocal electroretinography study. Retina 2007;27:159-64.
18. Boscia F, Furino C, Dammacco R, et al. Intravitreal triamcinolone acetonide in refractory pseudophakic cystoid macular edema: Functional and anatomic results. Eur J Ophthalmol 2005;15:89-95.
19. Karacorlu M, Ozdemir H, Karacorlu S. Intravitreal triamcinolone acetonide for the treatment of chronic pseudophakic cystoid macular oedema. Acta Ophthalmol Scand 2003;81:648-52.
20. Marmor MF. Hypothesis concerning carbonic anhydrase treatment of CME: Example with epiretinal membrane. Arch Ophthalmol 1990;108:1524-1525.
21. Cox SN, Hay E, Bird AC. Treatment of chronic macular edema with acetazolamide. Arch Ophthalmol 1988;106:1190-1195.
22. Fishman GA, Gilbert COT, Fiscella RG et al. Acetazolamide for treatment of chronic macular edema in retinitis pigmentosa. Arch Ophthalmol 1989;107:1445-1452.
23. Wolfensberger TJ, Chiang R, Takeuchi A et al. Inhibition of membrane-bound carbonic anhydrase enhances subretinal fluid absorption and retinal adhesiveness. Graefe's Arch Clin Exp Ophthalmol 2000; 238:76-80.
24. Arevalo JF, Garcia-Amaris RA, Roca JA, et al. Primary intravitreal bevacizumab for the management of pseudophakic cystoid macular edema: Pilot study of the Pan-American Collaborative Retina Study Group. J Cataract Refract Surg. 2007;33:2098-105.
25. Barone A, Russo V, Prascina F, et al. Short-term safety and efficacy of Intravitreal Bevacizumab for Pseudophakic Cystoid Macular Edema. Retina 2008 Oct 9. [ePub ahead of print]
26. Spitzer MS, Ziemssen F, Yoeruek E, et al. Efficacy of intravitreal bevacizumab in treating postoperative pseudophakic cystoid macular edema. J Cataract Refract Surg 2008;34:70-5.
27. Pendergast SD, Margherio RR, Williams GA, et al. Vitrectomy for chronic pseudophakic cystoid macular edema. Am J Ophthalmol 1999;128:317-23.
28. Peyman GA, Canakis C, Livir-Rallatos C, et al. The effect of internal limiting membrane peeling on chronic recalcitrant pseudophakic cystoid macular edema: A report of two cases. Am J Ophthalmol 2002;133:571-2.