Given the prevalence of angle-closure glaucoma around the world—less than that of open-angle glaucoma, but with a greater potential for causing blindness—increasing our understanding of the mechanisms that cause the disease should be a high priority. One individual who has done significant work in this area is Harry A. Quigley, MD, the A. Edward Maumenee Professor of Ophthalmology and director of the
Here, Dr. Quigley discusses the new theory, how it came about, and what his current research is revealing.
A Mystery to Solve
"About 10 years ago we began looking into the mystery of angle-closure glaucoma," he says. "The reason it's a mystery is that for every five individuals who ought to get this disease—because their eyes are small and their angles appear narrow on gonioscopy—only one gets it. Nevertheless, it's an extraordinarily common disease, especially among Asian people, and it causes blindness as frequently as open-angle glaucoma does because it's a more aggressive disease."
Ironically, he notes that the problem is not the lack of a treatment, but knowing which candidates with small eyes and narrow angles are likely to develop the problem. "Iridectomy is a beautiful, quick and easy procedure when it's done properly," he observes. "The problem is that we don't know who to proactively use the procedure on. Gonioscopy can tell us who has a narrow angle, but not who needs to have a hole made in the iris.
"This is an issue," he continues, "because as simple as iridectomy sounds, no treatment is totally benign. It's an intervention, and it may speed the development of cataract. Besides, implementing proactive treatment on a large scale in countries such as
Dr. Quigley says this situation inspired his search for a more predictive test. "The first step was to look at the characteristics of people who definitely have the disease," he says. "To do that, I worked with colleagues at the Singapore National Eye Center and Moorfields Eye Hospital in London, including Aung Tin in Singapore, Paul Foster and Gus Gazzard from London, and David Friedman here in the U.S. We realized that we had to measure something besides the anatomy of the angle, because measuring that in a static condition wasn't giving us the answer. So, we decided to do something that would cause the pupil to change shape, so we could see how the eyes responded.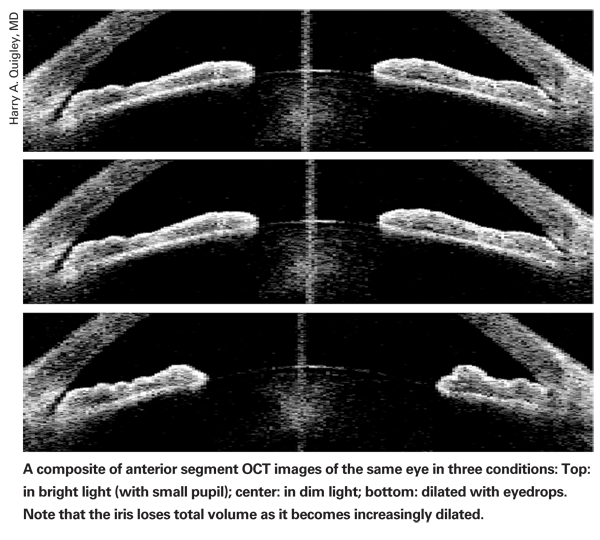
"First, we examined patients who walked into the hospital in
Dr. Quigley says their angle measurements did indeed reveal a difference. "When you contrast the two groups—just plain narrow but don't have the disease yet, and maybe never will, and the fellow eyes of those having an acute attack—there was a physiologic difference in how narrow the angle got in the dark," he says. "The angles in the fellow eyes of angle-closure glaucoma patients narrowed more in the dark than did the angles of people who didn't have angle closure. We realized that since these people started out with the same degree of narrowness, something about their anatomy or physiology of their eyes had to be different to account for the discrepancy between light and dark conditions." (The complete details of this study were recently accepted for publication in the Journal of Glaucoma.)
Explaining the Difference
To come up with a possible explanation for this, Dr. Quigley got together with David Silver, an engineer at the applied physics lab at Johns Hopkins who is an expert in fluid dynamics and volume and pressure inside the eye. "David and I had previously published some work on the dynamics of water moving between the iris and the lens," he explains. "He was an obvious choice to help understand what the iris is doing when the pupil dilates.
"One day we were looking at a geometric drawing of the front of the eye," he continues. "Suddenly, it occurred to us that if the iris maintained the same total volume when the pupil was dilated as when the pupil was small, everybody's iris would clog up their angles. David asked the key question: 'Is it possible that the iris can lose volume?' I noted that the iris is a very porous structure made of loose connective tissue. Unlike the cornea, if you hit the iris with the forceps during surgery, it falls apart. It's floppy. And researchers have known for years that the iris is highly permeable; even very large molecules or particles such as bacteria are found throughout the iris stroma almost instantly after they're injected into the anterior chamber.
"Given these facts, I realized that the iris has to act like a sponge," he says. "It's full of water, and when it dilates it very quickly dumps off that fluid into the anterior chamber. By losing volume, it avoids blocking up the angle. The iris tissue does go into folds, like drapery, but that just redistributes the volume that remains; it's not sufficient to explain why the angle isn't blocked every time the pupil dilates."
Finding the Right Tool
Dr. Quigley's desire to pursue the ramifications of this insight had to be put on hold for a simple practical reason: No current measuring technology provided enough information.
"The problem with gonioscopy is that you can't measure things in a quantitative way," he notes. "And, to be able to do what we wanted to do, we reasoned that we had to know what the pupil size was when the measurement was taken. If we didn't, we couldn't correct for it. That meant that the image or scan had to include at least half of the iris and pupil so we could measure the pupil size. That eliminated ultrasonic biomicroscopy as well, because its field of view is quite small."
It wasn't until three years later that an ideal measuring tool appeared: anterior segment optical coherence tomography. "The AS-OCT instrument that I've worked with, the Visante, is manufactured by Zeiss, for whom I am a consultant," he notes. "However, they just loan me the instruments; I'm not paid to do this work.
"Using the Visante, we were able to proceed with our initial research, measuring eyes in the light and the dark," he says. "What we found was that the iris loses half its volume going from a small pupil to a widely dilated pupil—and it does so in less than a second. In essence, this demonstrated that the normal iris is a sponge. So the next question was: What if individuals with angle-closure glaucoma have irises that are less spongy? If they don't release as much water, they'd be more likely to block up the angle. This is, in fact, what our current research is showing."
Dr. Quigley notes that they also considered the possibility that having an iridectomy might account for the difference. "Everybody we studied who was different in this respect had already had an iridectomy," he points out. "They weren't new cases. So we took about 10 patients and measured the change in volume both before and after iridectomy as a control for the experiment. The change in iris volume from light to dark conditions was the same before and after the surgery."
Asked what he thinks might account for the difference in sponginess they're finding in some eyes, Dr. Quigley says the connective tissue matrix makeup of the iris stroma is probably responsible. "It doesn't seem to correlate with eye color," he notes. "We think of this being a condition that's more prevalent in brown-eyed Chinese people, but African-derived people with brown eyes don't get any more angle-closure glaucoma than blue-eyed, European-derived people.
"The culprit may be the diffusional coefficient of the iris stroma—the density of the collagen fibrils and glycosaminoglycans and other components," he continues. "It's probably not caused by a border layer of cells that blocks fluid from moving in and out, although there may be a denser cellular layer on the surface of the iris which slows the diffusion of fluid.
"Whatever the cause," he notes, "the volume change itself is relatively stable; we imaged patients over a five-minute period, and once the initial change in volume is complete, the iris stops losing fluid. It doesn't continue to seep out as time passes."
Ramifications for UBM
Given that iris characteristics such as how its volume changes may be a key part of the angle-closure equation, simply measuring the angle may be less useful than previously assumed. "The biggest problem with ultrasonic biomicroscopy is that it's hard to get a wide view," observes Dr. Quigley. "Pupil size changes from second to second, and you need to know exactly how large the pupil was at the moment you measured the angle. People are still using UBM to measure just the last 500 microns where the iris is near the angle. We used to do that too, because it's what we were told was important. But in reality, UBM gives us too high a magnification of a process that's better seen if you back off a bit.
"Given this new information, much of the literature on UBM will have to be reevaluated, insofar as what it's doing is simply measuring the narrowness of angles," he continues. "The narrowness of the angle changes with pupil size. If you're not correcting for pupil size, that whole literature is suspect. UBM can also tell you the thickness of the iris in several places, but that's not sufficient to determine the iris's volume.
"Also, another variable that can cause trouble is that the UBM scan has to be taken directly through the center of the pupil," he notes. "If you tilt the instrument so the angle is oblique, your measurement will be altered significantly, and with the narrow field of standard UBM, you won't be able to tell that there's a problem. The anterior segment OCT avoids this problem because the patient's fixation determines exactly where the eye is. That makes it easy to get a shot straight through the center of the pupil."
Dr. Quigley notes that these issues don't eliminate the usefulness of UBM, however. "The disadvantage of the OCT technology is that it doesn't penetrate very far into the eye," he says. "You're using reflected light, so you can't see the ciliary body and you can't see the lens. In contrast, with UBM technology you can see all of those structures. So UBM will continue to play a very important role in the clinic."
What's Next?
Clearly, the realization that iris volume changes, and that the way it does may be a factor in angle-closure glaucoma, is a significant step forward in our understanding. Nevertheless, Dr. Quigley notes that this is not the end of the mystery. "This iris characteristic is almost certainly only one of a number of things that will influence the probability of developing angle-closure glaucoma," he says. "However, it's one that's measurable, and it's measurable noninvasively.
"Next, we hope to pursue this further with a prospective study," he continues. "We'll find a large group of individuals who might be candidates for angle-closure glaucoma because their angles are narrow under gonioscopy—people who aren't in need of immediate treatment, but could be at risk. We'll measure how they change from light to dark. Then we'll treat half of the eyes with iridectomy and follow them carefully for five years; we'll see whether this characteristic successfully predicts which individuals develop signs that they're going to undergo angle closure, and if so, which specific parameters for iris volume change are the predictive ones."
Dr. Quigley says it's still too early to make clinical recommendations relating to this development. "We don't have enough data yet to suggest that clinicians should be measuring these changes," he says. "However, once we've done our prospective study I think we'll be able to offer some guidelines. Also, the software for doing this is not presently part of any anterior segment OCT instrument. For that reason, I suspect that initially, once sufficient supporting data has come in, patients will be referred for evaluation to a center that has anterior-segment OCT with the necessary software, just as we used to do when patients needed a visual field but few clinics had the instruments. Hopefully, if our data continues to support this approach, companies like Zeiss will incorporate software for this purpose into their instruments."
Further down the line, Dr. Quigley says he and his colleagues hope to study the impact of choroidal swelling on angle closure—a factor discussed in his 2003 article. Whatever data all of these investigations turn up, it seems clear that Dr. Quigley and his colleagues are making significant strides towards solving the angle-closure glaucoma mystery.
Quigley H, Friedman DS, Congdon NG. Possible mechanisms of primary angle-closure and malignant glaucoma. J Glaucoma 2003;12:2:167–180.