Glaukos iStent
The Glaukos iStent, like many MIGS devices, can be implanted during cataract surgery in the hopes of lowering intraocular pressure. Robert Noecker, MD, an ophthalmologist based in Fairfield, Conn., explains the optimal use of the iStent. “The best use is in allying it with the on-label indication, for mild to moderate glaucoma and those who need cataract surgery,” he says. “In my experience, this is where it works well—for patients with relatively low pressures, or when we’re being opportunistic for cataract surgery.”
Dr. Noecker says that one advantage of the iStent is that surgeons are able to implant more than one device to further lower IOP. “It’s a reasonable thing to do because there’s a fair amount of data to suggest additive IOP lowering,” he says. “If we can place these devices near collector channels, there’s a higher chance we’ll hit a great area to enhance.” However, Dr. Noecker notes that reimbursement is still an issue when implanting more than one device. “The biggest barrier for this is reimbursement,” he says. “I have done it separate from cataract surgery, and the patient has to pay for that portion because their insurance doesn’t always cover it. So it depends on whether the patient wants to pay for another iStent.”
In terms of implanting the device, Dr. Noecker provides some surgical pearls to maximize positive outcomes. “It’s really all about the setup and patient selection,” he says. “I also tend to use a fair amount of viscoelastic to keep the cornea clear and help make the trabecular meshwork angle more visible. The biggest error I see when teaching residents is that they tend to under-turn the head and microscope. You need a good point of attack so that the meshwork is almost vertical to make it a nice, easy, flat target. My motto is, in terms of visualization or tilting the head, it’s hard to do it too much.”
Once the device is implanted, Dr. Noecker offers some advice. “I always make sure I touch the meshwork with the blunt side of the stent. This helps you make sure you’re deep enough, and it also indents the meshwork forward a bit,” he says. “I think that makes it much easier to hit the target.”
When discussing postop complications, Dr. Noecker describes what to expect and how to react. “Honestly, complications are rare once you become familiar with the procedure. During the first three months of familiarizing yourself with the procedure, the complications usually come from surgeon error,” he says. “The most common thing to worry about is, when you hit the canal, having your view obscured by blood. If a patient is on a blood thinner, this can be a bit of a problem. It’s like striking oil—you feel good seeing it because it means you hit the target. It can get excessive, though.
“Obviously you can under-implant the stent,” he continues. “When I’m teaching surgeons, I think that’s an error. They’re anxious to let go of the device before it’s implanted. When you implant the iStent, there’s a natural rotation of the eye that occurs, so you want to rotate the eye back to a natural position so there’s no pressure on the injector. If there is pressure, the injector can flip the stent out of a good position.”
Despite these concerns, Dr. Noecker is satisfied with the iStent. “In terms of few complications, I like the iStent,” he says. “The chance of some really bad stuff happening is minimal. It’s one of the lowest-risk glaucoma procedures that we do.”
However, Dr. Noecker notes some reservations about the iStent, and what he would like to see change. “There’s a real learning curve for implanting the device. Once you have it in a good position, you’ve nailed it, but achieving that can be difficult. The need to do intraoperative gonioscopy is limiting,” he says. “Sometimes patients don’t have a clear cornea to look through, and this can impair the viewing or placement of the device. So getting away from using intraoperative gonioscopy would be ideal.”
Reimbursement is moderate, Dr. Noecker claims. “In other markets, I’m sure it’s reimbursed well, but, for me, it’s moderate,” he says. “It doesn’t pay as much as doing cataract surgery. The coverage has become very good in the past few years, and almost all payers recognize it and pay for it, but how much is variable depending on your market.”
Alcon CyPass
Approved in late July 2016, Alcon’s CyPass has proved to be another contender in the arena of MIGS devices. The device was approved in the United States based on results from the COMPASS clinical trial, and has been studied in clinical trials in Europe since 2009. Tsontcho Ianchulev, MD, MPH, of San Francisco, shares his experience using the CyPass.
In terms of patient selection, Dr. Ianchulev says that to ensure the best outcomes, it pays to follow the FDA guidance. “We try to treat those patients with mild to moderate glaucoma who are undergoing cataract surgery,” he says. “Diligent patient selection is essential for the best outcomes. You don’t want to go for end-stage or severe cases, which need more drastic IOP lowering. If you do it for patients with cataracts, you help those patients stay medication-free for two years and beyond.” In the COMPASS clinical trial, 61.2 percent of 374 patients maintained mean IOPs between 6 mmHg and 18 mmHg at two years.1
Dr. Ianchulev also provides some advice for the procedure itself to maximize positive outcomes. “You’ll definitely have a learning curve with this procedure,” he avers. “It’s probably five cases before you get the hang of it. It’s a straightforward surgical procedure, but it’s not trivial. As with other MIGS, you have to have some gonioscopy skill, be able to visualize the angle and implant the device correctly. Experience and practice make for the best outcomes.”
Though the CyPass proved safe and effective in its clinical trial, you can still run into some problems. “The complications are generally mild, tolerable and, ideally, resolvable over time,” Dr. Ianchulev says. “You have a little bit of inflammation, and in some cases you can have some reflux bleeding. None of it is all that consequential or interferes with the results we want.”
In the COMPASS clinical trial, only 5.3 percent of the CyPass group (20 patients) underwent secondary ocular surgeries. Corneal edema associated with the surgical procedure resolved within the first postop month in 98 percent of CyPass group subjects. No cases of retinal detachment, pupillary block, endophthalmitis or hypopyon were reported during the study.1
Dr. Ianchulev also shares his thoughts on some ways to improve the procedure. “The device is not the easiest one to implant because visualizing the meshwork can be difficult, versus being able to implant it into the superciliary space,” he says. “But I think it’s pretty much where it ought to be compared to the other available devices. It’s got a great learning curve surgically. On the other hand, we could improve the procedure by having it move towards treating the moderate-to-severe glaucoma population. Having a solution for trabeculectomy is where there is definitely room for development.”
When discussing reimbursement for the CyPass, Dr. Ianchulev says, “It has its own CPT code, and Medicare will start paying for it if they haven’t already. It’s FDA-approved, so the code will be in the same ballpark as the other MIGS devices. It’ll probably be reimbursed higher than cataract surgery. When it becomes fully reimbursable, it will definitely justify the clinical effort.”
Allergan XEN Implant
Allergan’s XEN Implant can be used either alone or in conjunction with cataract surgery to treat patients with mild to moderate glaucoma. Joseph Panarelli, MD, an ophthalmologist from New York, offers his experience using this implant. Dr. Panarelli describes his patient selection as an integral part of ensuring good outcomes. “The XEN implant can be used for a variety of patients as it tends to be a safe, rather straightforward procedure that produces a diffuse, more posteriorly directed bleb. I think we’ll see that these blebs are at less risk for leaks, as well as infectious complications,” he says. “I would consider using it in patients with mild to moderate glaucoma as either a standalone procedure or in combination with cataract surgery. I would even consider using it in ocular hypertension patients who are intolerant of medical therapy. But I hesitate to use this device in those with advanced disease, as it’s difficult to
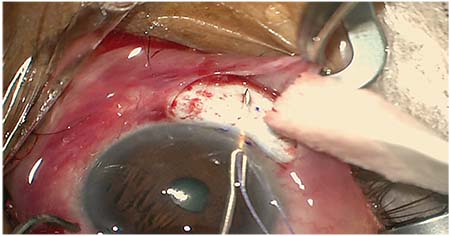 |
| Implanting the Allergan XEN implant: “With this procedure, your first shot at delivering the device is your best one,” says New York’s Joseph Panarelli, MD. |
In describing the implantation procedure, Dr. Panarelli notes that he has yet to implant more than one device. “The implant is inserted through a clear corneal, temporal wound,” he says. “We often target the nasal quadrant and, if the anatomy allows, try to aim more for the 12 o’clock position. Placing the device in the temporal quadrant is not technically possible unless you alter your approach. We generally avoid placing blebs in the inferior quadrant—though this may change. Hence, one implant per patient is my general approach. A second device could be placed a few clock hours away in the same quadrant if the first becomes encapsulated and IOP is not optimally controlled, but the device has not been utilized in this way yet.”
Dr. Panarelli offers surgical pearls that he’s gathered from his firsthand experience implanting the device. “With this procedure, your first shot at delivering the device is your best one,” he says. “When first learning to perform the procedure, I recommend doing a retrobulbar block or at least a peribulbar block. Minimizing patient movement and discomfort is extremely helpful in these cases. I also recommend visualizing the angle structures with a gonioprism after the injector is in position to get a feel for where the stent will go. The stent should be seated at or above the trabecular meshwork. Delivering the device too posteriorly will result in it being obstructed by the iris. I prefer to aim as anterior as possible, as I’m not worried about endothelial issues, given the small size of the implant and the fact that it barely protrudes into the anterior chamber if placed correctly.
“The most common issue is improper device placement, either leaving the implant too far outside the eye or too much inside the eye,” Dr. Panarelli continues. “The stent is 6-mm long and should be positioned as follows: 2 mm of the stent in the subconjunctival space, 3 mm intrascleral and 1 mm in the anterior chamber. Delivering the device is a challenge at first as it is unlike any other procedure, but with practice it can become routine.”
Because this procedure is unique and relatively new, Dr. Panarelli offers some tips on how to deal with common intra- or postop complications. “If too much of the implant is sitting outside of the eye, it’s the result of exiting the sclera too anteriorly,” he explains. “This is not ideal as the stent can end up taking a more curved course or become kinked. If the implant is too long inside the eye, it’s likely because the device migrated back into the eye when the stent was being delivered. Gentle forward pressure is required during device delivery. Once the stent hydrates, it’s rather difficult to grasp and advance farther outside the eye. Often, it must be removed and the procedure started again. Bleeding becomes an issue at this point, as there is often reflux blood at the site of the prior attempt. A cohesive viscoelastic should be used to tamponade that area and a new pass should be made in an adjacent area.”
Because of the relatively recent approval of the device (November 2016), physician reimbursement is an important topic with the XEN. Dr. Panarelli says, “Currently, prior authorization needs to be obtained before XEN Gel Stent placement. Patients can pay out of pocket, and the claim can later be submitted for reimbursement. As with all new devices, this can be a challenging process at first.”
Trabectome
Trabectome stands alone among the original MIGS procedures, as there is no device to implant. The procedure consists of the tip of the Trabectome removing a strip of the trabecular meshwork via microelectrocautery, increasing flow into the natural drainage system. This is done through the same corneal incision used in cataract surgery. Brian Francis, MD, MS, shares his experience and advice with this unique procedure.
As with the other surgeries, Dr. Francis emphasizes the importance of diligent patient selection in order to get the best outcomes. “The one advantage of the Trabectome is that it can be used as a combined procedure for cataracts and glaucoma treatment,” he says. “We sometimes use it to treat severe glaucoma because it’s all just based on target IOP. Anyone with open-angle glaucoma, primary steroid-induced, etc., can benefit from the procedure if we can hit their target IOP.
“Preoperative planning is essential to the success of the procedure, and this includes decent, consistent patient selection,” Dr. Francis continues. “You just have to take the proper precautions prior to surgery. Intraoperatively, when I make my incision into the eye, I draw blood out so I can better see the target tissue. This tip basically applies to every MIGS. One of the most difficult parts of the Trabectome procedure is knowing when you’re in. You have to make sure you have a good angle. Practice helps with this. You want to make sure you’re not too deep, so sometimes you want to pull back to make sure you’re not too far into the eye.”
In terms of postop
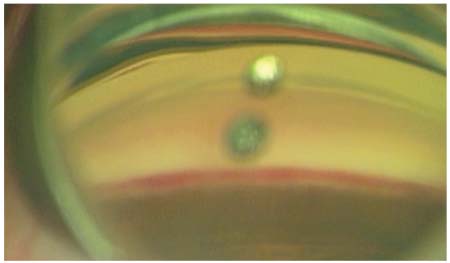 |
| Trabectome, after blood reflux. The procedure consists of the tip of the Trabectome unroofing the trabecular meshwork, increasing flow into the aqueous drainage system. |
In a clinical study that evaluated the results of Trabectome for open-angle glaucoma, postop complications were minimal. Early IOP spikes, defined as IOPs >10 mmHg above baseline within one week after surgery, occurred in four eyes (4.9 percent). Hyphema was observed in 19 eyes (23.3 percent). Hyphema was minimal and disappeared without any special management one to 10 days after surgery.2
Dr. Francis describes the reimbursement for Trabectome as more than satisfactory. “It’s good,” he says. “It’s been steady ever since it’s had a regular CPT code. There are actually two reimbursement codes that can be used: The trabeculectomy code or the gonioscopy code. Surgeons just use whichever one they prefer. I think the reimbursement is slightly different, but it’s consistent.”
The biggest issue with the procedure is purchasing the equipment, says Dr. Francis. “When I talk to other surgeons, the biggest barrier with Trabectome is startup costs,” he says. “Typically, the surgery center will prefer other methods, so it’s a pain to try to convince them otherwise. Hopefully there will be programs soon that minimize startup costs.”
The Kahook Dual Blade
In terms of newer MIGS devices, the single-use Kahook Dual Blade appeals to surgeons because of its flexibility in treating different patient types. The KDB procedure consists of passing the blade through a clear corneal incision and using it to make two parallel incisions in the TM and the inner wall of Schlemm’s canal.
Malik Kahook, MD, the creator of the device, gives his advice on how to get the best results. Again, it all begins with patient selection, he says. “A typical patient is one who has co-existing cataracts, with mild to moderate glaucoma,” he explains. “The goal is to decrease dependence on topical medications as well as to lower intraocular pressure. Another group of patients includes those who are already pseudophakic and can benefit from a reduction in both medications and IOP. Patients with chronic angle closure glaucoma, pseudoexfoliation and pigmentary glaucoma seem to do particularly well with KDB treatment.”
Dr. Kahook provides some intraoperative tips. “Learning how to do intraoperative gonioscopy is key,” he says. “For surgeons who are doing ab interno angle procedures already, picking up KDB will be a smooth transition with only minor differences compared to other angle-based approaches. I always recommend that surgeons practice goniocopy intraoperatively on five to 10 cases before attempting their first angle procedures. I recommend using a cohesive viscoelastic to deepen the angle and maintain the anterior chamber. The surgeon should be careful not to press too hard with the lens since this causes corneal striae and will obstruct your view.
“Other pearls for use include positioning the head of the device against the anterior wall of the canal without lifting up, which allows the ramp on the KDB to do the job of lifting up the trabecular meshwork so the blades create parallel incisions,” he continues. “At the end of the case, the surgeon should hydrate the wounds very well and keep the IOP at the high-normal range to encourage outflow of aqueous through the collector channels and out of the eye.”
Dr. Kahook also shared his experience with the Dual Blade’s intraoperative and postop complications. “The most common observation at the time of KDB treatment is reflux of blood from the collector channels that have been exposed by removal of the diseased trabecular meshwork,” he says. “This is a good prognostic indicator that the treatment will result in measurable reduction of IOP postop. It is rare to have retained blood postop day one, and, in my experience, patients have very quick visual recovery on par with standalone cataract surgery.” In a study that evaluated the effects of the Kahook Dual Blade on trabecular meshwork, across 157.5 ±26.3 degrees the procedure resulted in a decrease of IOP from 18.3 ±1.7 mmHg to 11.3 ±1.0 mmHg (p <0.01)3.
Ab Interno Canaloplasty
Another new MIGS procedure is ab interno canaloplasty, which targets a population similar to the other MIGS devices. It’s best utilized for those patients with mild to moderate glaucoma with elevated IOP.
Dr. Panarelli offers his insights into this procedure. “ABiC is considered to be a MIGS procedure as it can be performed through a microincisional approach (1.8-mm clear-corneal wound), is minimally traumatic to the targeted tissue and has reasonable efficacy and a favorable safety profile,” he says. “When performing the procedure, the surgeon uses an illuminated microcatheter to first thread Schlemm’s canal 360 degrees and then viscodilate that space as the catheter is withdrawn.”
In a case series review of 122 eyes with a baseline IOP of 18.6 ±6.4 mmHg, mean IOP was reduced by 28.49 percent at six months (n=32). At six months, over half of the study population (n=17) were medication-free, with a mean IOP of 12.1 ±2.1 mmHg.4
“ABiC is an appealing new procedure, as it is conjunctiva-sparing, and one should encounter less bleeding when compared to similar procedures such as gonioscopy-assisted transluminal trabeculotomy and other angle-based procedures,” Dr. Panarelli continues. “The biggest downside is the considerable learning curve that the surgeon faces, in terms of performing intraoperative gonioscopy as well as operating in the angle to locate Schlemm’s canal. Like so many other new procedures, future prospective, randomized trials comparing ABiC to more traditional procedures will be needed to determine its true efficacy and its role in the glaucoma surgeon’s armamentarium.” REVIEW
Dr. Noecker is a consultant and clinical investigator to Glaukos. Dr. Ianchulev is a consultant for Alcon. Dr. Panarelli is a consultant to Allergan, Aerie and has received an honorarium from Glaukos. Dr. Francis is a Neomedix surgical trainer. Dr. Kahook is a consultant for Alcon and Allergan. He receives patent royalties from ClarVista Medical, New World Medical, J&J Vision and ShapeTech.
1.https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150037B.pdf accessed 10 Apr 2017
2. Mizoguchi T, Nishigaki S, Sato T, Wakiyama H, Ogino N. Clinical results of Trabectome surgery for open-angle glaucoma. Clin Ophthalmol 2015;9:1889-1894.
3. Seibold LK, Soohoo JR, Ammar DA, Kahook MY. Preclinical investigation of ab interno trabeculectomy using a novel dual-blade device. Am J Ophthalmol 2013;155:524-529.
4. http://ascrs2016.abstractsnet.com/handouts/pdfs/22999-0094.pdf accessed 23 May 2017