Retinal prosthesis technology is currently under development in more than 13 centers across five continents worldwide. Consequently, there are significant differences in the technology and stages of development across groups.
Patients with advanced retinal degenerative diseases and their family members often actively seek information related to the latest neuroprotective treatments to slow their disease progression or rehabilitative technologies that may restore their sight. Retinal physicians are faced with the task of explaining to patients the state of retinal prosthesis technology, when it will become practical and what level of vision might be restored. While this may seem daunting, a systematic approach to categorizing retinal prosthesis technology greatly simplifies an understanding of the field.
Retinal Prosthesis Types
A retinal prosthesis is a device that gathers visual information with an electronic detector, then processes and converts that image into a form of stimulation that the retina and brain can interpret as a form of vision. All retinal prostheses can be categorized according to three fundamental design features—detector type, stimulation type and stimulation site.
Detector Type
Detectors for retinal prostheses can vary from the CMOS or CCD chips found in webcams or camcorders to microphotodiodes that act as tiny solar cells, turning light directly into electrical current. This distinction is important because CMOS and CCD devices require an external power supply, and microphotodiodes do not. Consequently, at the present time, CMOS or CCD cameras are mounted to eyeglass frames, external to the body. They are used to collect video images that are, in turn, processed by a small computer worn near the waist. This processor identifies important features of the visual environment such as object edges and sends a reduced form of the image to a receiver/stimulator "chip" implanted within the body that receives the wireless transmission and in turn stimulates the retina according to the device stimulation type.
Microphotodiodes, however, are often im-planted within the eye and rely upon the cornea and lens to focus the light upon them. Patients with these implanted sensors can be refracted to improve device performance. In addition, since microphotodiodes are manufactured on a microscale, they are often each integrated with their own stimulation site. Consequently, retinal prostheses employing these devices often comprise thousands of stimulators as compared to systems that rely on external detectors that presently employ fewer than 100 stimulators. In addition, while systems that use eyeglass-mounted cameras require patients to scan their environment using head movements, implanted microphotodiode array systems allow patients to employ natural eye movements and higher level cortical circuits to redirect their visual attention.
Stimulation Types
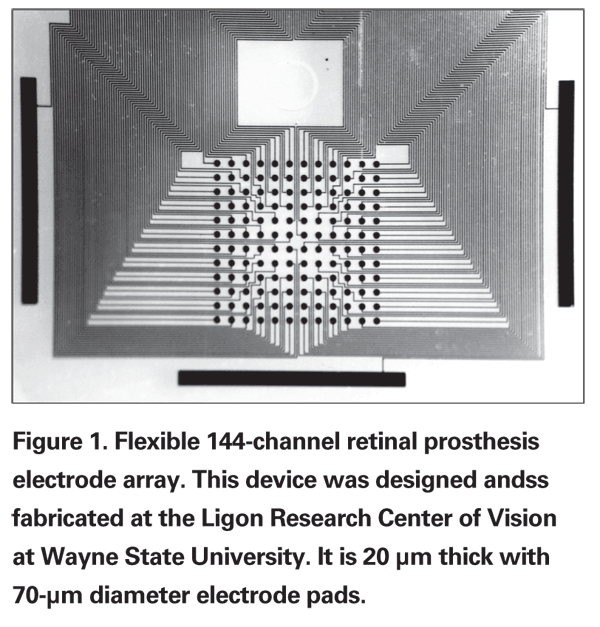
• Electrical Stimulation. The vast majority of retinal prostheses in development today employ electrical current to stimulate the retina, producing electrophosphenes. These are small, round, oval or elongated spots of light produced when pulses of electrical current are applied to the retina.1-3 This form of stimulation was originally employed in cochlear implants that deliver electrical current via multiple linearly arranged electrodes that stimulate the cochlea to restore hearing. The challenge faced by retinal prosthesis designers is the fact that significantly greater amounts of data are conveyed visually, creating a bandwidth bottleneck. Since electrode arrays are arranged as two-dimensional matrices (See Figure 1), significantly more electrodes are required to restore vision than to restore hearing. In addition, to achieve better visual acuity, electrodes must be small in diameter and closely spaced. Small diameter electrodes have a greater tendency to break down water into hydrogen peroxide and may fail during stimulation when greater amounts of electrical current are needed. This can occur in cases where the retinal degeneration is severe or when the electrode array is not in close apposition to the retina.
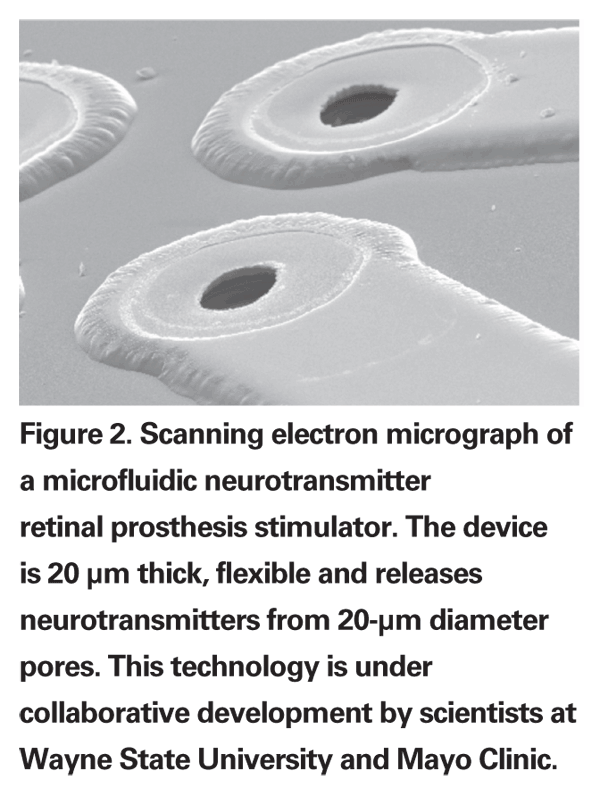
• Neurotransmitter Stimulation. In an effort to develop a more naturalistic method of retinal stimulation, the collaborative groups at
Stimulation Site
• Epiretinal. The most critical component of the retinal prosthesis system is the tissue interface formed at the junction of the neurosensory retina and the stimulator. Retinal prosthesis designers interface to either the epiretinal surface or the subretinal surface, for a variety of reasons. Epiretinal devices are placed in very close proximity to the internal limiting membrane, where retinal ganglion cells may be stimulated directly or indirectly via bipolar cell synapses. Epiretinal placement is achieved after pars plana vitrectomy with removal of the posterior hyaloid. Fixation is via a retinal tack. The greatest challenge associated with epiretinal placement and fixation is assuring that the stimulator is in very close proximity to the retina over the entire extent of the stimulation site.5,6 Otherwise, large areas of the visual field covered by the prosthesis will remain non-functional. Close apposition between the stimulator and the retina may be difficult in cases where the stimulator cable introduces mechanical torque at the tissue interface. Epiretinal stimulators are less surgically complex to place and remove as they do not require surgical detachment of the retina and transchoroidal subretinal insertion.
• Subretinal. Subretinal placement assures close proximity to the neurosensory retina and obviates the need for tack fixation as the retina is draped over the stimulation sites. The surgical approach is more complex, however, requiring a pars plana vitrectomy, formation of a localized retinal detachment bleb and trans-scleral, trans-choroidal incisions for the ab-externo insertion of the stimulator into the subretinal space. After insertion, the retina is re-attached via internal tamponade.7
Fine adjustment of device position is more difficult as well, since the device cannot be physically handled from within the eye as it remains subretinal, without a retinotomy. In addition, since the retinal stimulator is placed between the neurosensory retina and the retina pigment epithelium, a chronic localized retinal detachment is created immediately underlying the tissue interface. This can worsen the retinal degeneration at the tissue interface, and may ultimately lead to system failure. Subretinal devices are also prone to subretinal proliferative vitreoretinopathy with the formation of a tissue capsule, enveloping the device.
Patient Selection
The basic operating principle be-hind all retinal prostheses relies upon the fact that while advanced stages of retinitis pigmentosa and age-related macular degeneration induce profound photoreceptor cell loss, the inner retinal processing layers remain functional, but without an afferent input.8 Thus, the retinal prosthesis is a sensory substitution device, designed to substitute the lost natural visual input provided by photoreceptors with another form of stimulation that the remaining retina can process and send to the brain as a visual signal. Physicians and patients must be aware, however, that not all patients with advanced retinal degeneration are candidates for a retinal prosthesis.
The retinal prosthesis is a computer-brain interface. In order for vision to be conveyed to the brain, the device must be capable of properly encoding vision into a stimulation format that the retina can process and send to the brain. Thus retinal prosthesis stimulators rely upon on a healthy inner retina to communicate with. Therefore, diseases that disorganize, degenerate or scar the inner retina, such as trauma, advanced diabetic retinopathy and retinal detachment, are not current candidates for treatment with a retinal prosthesis.
In addition, patients with optic neuropathies are not candidates for retinal prosthesis, as the technology relies upon a healthy optic tract to convey retinal reponses to the brain. Histologic and electrophysiologic evidence in animals and humans has shown that a small subset of patients with the most severe forms of retinitis pigmentosa have lost their inner retinal function and become insensitive to electrical stimulation.9 Consequently, prior to enrollment in retinal prosthesis clinical trials, RP patients undergo screening studies in which small electrical currents are applied to the surface of the eye to assure that their retinas are capable of producing phosphenes in response to electrical current.
Functional Performance
Clinical trials with chronic or semi-chronic (one-month) implant durations are currently under way in the
Retinal prosthesis technology has been in development for more than two decades. Early devices are currently in clinical trials and are demonstrating exciting results. A systematic approach to fundamental retinal prosthesis components will help foster greater understanding of the field.
1. Humayun M, Propst R, de Juan E Jr., McCormick K, et al. Bi-polar surface electrical stimulation of the vertebrate retina. Arch Ophthalmol 1994;112:110-116.
2. Humayun MS, de Juan E, Jr., Dagnelie G, Greenberg RJ, et al. Visual perception elicited by electrical stimulation of retina in blind humans. Arch Ophthalmol 1996; 114:40-46.
3. Rizzo JF 3rd, Wyatt J, Loewenstein J, Kelly S, Shire D. Methods and perceptual thresholds for short-term electrical stimulation of human retina with microelectrode arrays. IOVS 2003:44: 5355-5361.
4. Finlayson P, Iezzi R. Glutamate Stimulation of Retinal Ganglion Cells in
5. Basinger BC, Rowley AP, Chen K, Humayun MS, et al. Finite element modeling of retinal prosthesis mechanics. J Neural Eng 2009:6:55006.
6. de Balthasar C, Patel S, Roy A, et al. Factors affecting perceptual thresholds in epiretinal prostheses. IOVS 2008:49:2303-2314.
7. Besch D, Sachs H, Szurman P, et al. Extraocular surgery for implantation of an active subretinal visual prosthesis with external connections: Feasibility and outcome in seven patients. Br J Ophthalmol 2008;92:1361-1368.
8.
9. Kent TL, Glybina IV, Abrams GW, Iezzi R. Chronic intravitreous infusion of ciliary neurotrophic factor modulates electrical retinal stimulation thresholds in the RCS rat, IOVS 2008;49:372-379.
10. Humayun MS, Dorn JD,
11. Caspi A, Dorn JD, McClure KH, Humayun MS, et al. Feasibility study of a retinal prosthesis: Spatial vision with a 16-electrode implant. Arch Ophthalmol 2009; 127:398-401.