Protecting the integrity of the cornea, specifically the endothelium, is one of the priorities of the cataract surgeon. To this end, the biocompatibility of chondroitin sulfate (CS) and hyaluronic acid (HA), along with their ability to coat and adhere to surfaces, make them natural choices for ophthalmic viscosurgical devices for protecting the cornea and maintaining space during phaco and IOL implantation. We'll look at how each chemical serves the needs of ophthalmologists and patients.
The Two Agents
Chondroitin sulfate and hyaluronic acid, along with heparin sulfate, are actually the primary components of the vitreous humor.1,2 In contrast to the gelatinous vitreous, the aqueous is a true liquid, composed of mostly water, with some salt, ascorbate and glucose.3 The aqueous is constantly produced and drained in order for the eye to maintain the best optical clarity, shape and pressure, and it provides nutrients to the anterior portion of the eye. During cataract surgery, however, it's necessary to maintain space and protectively coat the endothelium in order to ensure the best visual outcomes. HA was first used for this purpose during cataract extraction in the late 1970s, and Pharmacia's Healon (sodium hyaluronate) became the first viscoelastic product around that time.4 CS was introduced in Alcon's Viscoat soon after. The benefits and properties of each of these substances can minimize postoperative recovery time and optimize visual outcomes.
The classification of OVDs revolves around their rheologic characteristics, specifically their viscosity and corresponding retention in the anterior chamber during surgery. Modification of the individual rheologic traits has produced a market of OVDs with an array of biomechanical properties. Most of the currently available OVDs contain HA, CS or both—the foundation of an OVD's properties.
Defining the Molecules
HA is important in extracellular matrix construction and is found in cartilage and in the synovial fluid that lubricates the joints; some postulate that injections of hyaluronic acid temporarily help reduce the friction of the worn joints of osteoarthritis. The chemical's therapeutic benefits are negligible when dosed orally, as stomach acid breaks down the molecule, but it has been used with anecdotal success when injected directly into the affected joint. Its first widespread commercial use was in veterinary medicine, and it's been used for decades in the form of viscosupplementation. It appears that HA injections suppress the synthesis of inflammatory cytokines and of cartilage-degrading proteinases, which are of particular concern in worn and damaged joints. In humans, controlled studies yield conflicting results as to its measurable effects but suggest a similar degree of efficacy.5
CS is a ubiquitous glycosaminoglycan that is also found in connective and cartilaginous tissues throughout the body, whose structure and precise role varies with species and tissue.6 It's involved in the clinical progression of osteoarthritis: As CS degrades and the cartilage loses its ability to resist compression, joint pain and swelling occur. Semisynthetic preparations of chondroitin, derived from bovine trachea, have been used in humans and horses since the 1960s to treat arthritic conditions, although the mechanism of action has never been fully or convincingly explained.7 Today, chondroitin is manufactured from bovine, porcine or shark cartilage.8 Since the structure of CS varies, so does the content of chondroitin supplements, depending on the species of its derivation.9 This likely accounts for at least some of the differences in the research reports regarding its ability to reduce the pain and swelling associated with arthritis.
HA and CS also appear in the vitreous. Hyaluronan is concentrated in the posterior section of the vitreous, with an average molecular weight in humans of 2 to 4 million Da. On its own, hyaluronan chains don't appear to form strong, stable entanglements.10 Unlike other glycosaminoglycans, which are synthesized within the Golgi apparatus, HA is synthesized by several enzymes on the surface of the plasma membrane of the Golgi apparatus.1
Chondroitin has been known to be a primary component of the vitreous for 50 years,11 although it was first isolated from cartilage tissues in 1861.12 Two chondroitin sulfate proteoglycans are found in the vitreous: Type IX collagen and versican. The latter is more abundant, has a molecular mass greater than 1,000 kDa, and seems to link the hyaluronan network to other parts of the vitreous structure.10
Easing IOL Insertions
HA was first isolated from the vitreous; it derives its name from the Greek term for the vitreous humor. In the late 1960s, when vitrectomy was popularized by retinal surgeons for vitreous opacification, traction, hemorrhage, diabetic retinopathy, retinal detachment and macular holes, HA was the obvious choice of Charles Schepens to replace the missing gel. Claes Dohlman, of the Schepens Eye Research Institute, also used HA to plug leaky anterior chambers. It was therefore fitting that when I was a fellow studying corneal graft rejection in rabbits and encountering a persistent problem of leaky anterior chambers, David Miller, MD, suggested HA. I tried it, and it worked, both to stop leaks and to preserve the clarity of the corneas. Schepens researcher Endre Balazs, MD, discovered a way to purify HA from umbilical cord and rooster combs, and licensed it to Pharmacia.
During the late 1970s through the 1980s, research focused on finding the optimum coating for IOLs, so as to protect the corneal endothelium from damage during insertion. Multiple substances, including polyvinylpyrollidone, HA and CS, were investigated for their ability to absorb mechanical forces and to minimize electrostatic interaction.13
While Healon is effective at maintaining space and protecting the endothelium, an OVD cannot be expected to do everything optimally.
Many surgeons working with HA noted transient postoperative intraocular pressure spikes, perhaps related to the size of HA's molecular chains, which temporarily slowed trabecular meshwork drainage. An interest in finding an OVD that more strongly adhered to the IOL, as well as a desire to minimize postoperative IOP spikes, fueled research into the ideal OVD.
Researchers turned their attention to CS, which carries an extra negative charge that allows it to more thoroughly coat the positively charged IOL and reduce the electrostatic interaction between the IOL and endothelium. At the time, research dictated that if left inside the anterior chamber, chondroitin cleared in less than 40 hours,13 in comparison to HA, which took up to 14 days.14 But typically, today, most of the OVD is cleared from the chamber at the close of the case. Comparison studies amongst various OVDs demonstrate that ocular hypertension due to OVDs is essentially the same regardless of which OVD is used, and typically resolves within 24 hours.15
Comprehensive Protection
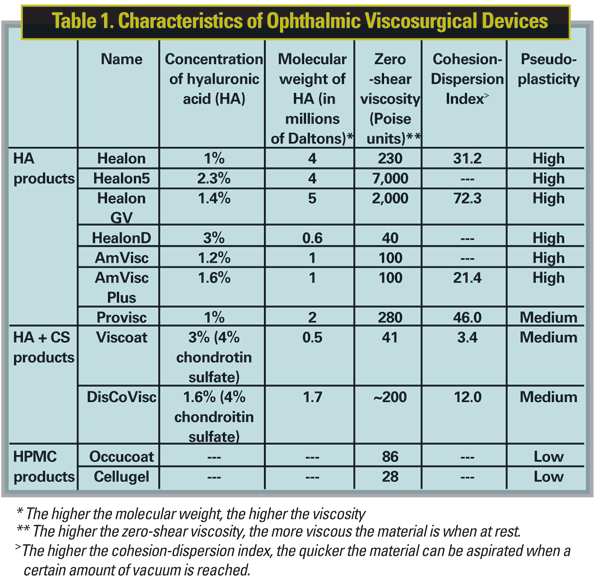
A lot is expected from an OVD during cataract surgery: It must provide mechanical protection by maintaining space in the anterior chamber; spread out to protect the endothelium; be retained during the dynamics of the lens-removal processes; be pliable enough to accept the lens as it's being inserted and be easily and completely removed at the end. The interaction of the following rheologic properties creates the characteristics specific to each OVD. (For the measurements of the key properties in today's OVDs, see Table 1)
• Viscosity and shear. This is a measure of resistance to flow. High-viscosity solutions tend to stay within the anterior chamber and separate tissues well. Shear is defined as the stress that's applied parallel to the material. If the material maintains its viscosity regardless of the rate of applied shear, it's known as Newtonian. Examples of Newtonian fluids are CS and water. If the material become less viscous as shear rate increases, it's pseudoplastic.
In practice, when force is applied to the syringe during injection of an OVD, OVDs with high pseudoplasticity, such as Healon, become less solid. Highly pseudoplastic cohesives don't require wide cannulas and are more easily injected.16 Pseudoplasticity, however, is not directly related to retention during surgery. The corneal endothelium contains HA receptors,17 so retention of the OVD is related to the presence of HA. OVDs with CS, such as the dispersive Viscoat, have an extra negative charge, and are therefore better retained within the chamber.18 This retention is important for endothelial protection; however, such lower-viscosity, dispersive OVDs are also more difficult to remove, and prolonged aspiration may increase en-dothelial damage.
• Elasticity. Following compression or stretching, elastic materials will return to their original shape. During cataract surgery, materials that are viscoelastic lubricate tissue, maintain space and absorb the vibrations created during phacoemulsification.
• Cohesiveness. This is a function of molecular weight and elasticity. The more cohesive the OVD, the lower the flow rate, so cohesive OVDs—of which Healon GV is one—are good for space maintenance and are more easily removed as a bolus during irrigation and aspiration.
• Surface tension, contact angle and molecular charge. The ability of an OVD to coat the endothelium and IOL is related to its surface tension, contact angle and molecular charge. Contact angle is literally the angle at which a liquid meets a solid surface. Low surface tension, low contact angles and more negatively charged OVDs better coat the endothelium, IOL and instruments. CS solutions (alone or in combination with HA) and hydroxy propyl methylcellulose solutions have lower surface tension and contact angle values than solutions of HA alone.16
• Cohesion-Dispersion Index. The CDI is defined as the percentage of viscoelastic agent aspirated/100 mmHg; it more comprehensively classifies OVDs in terms of viscosity, cohesion and dispersion.19 This new classification system also accounts for the relatively recent introduction of viscoadaptive (Healon 5) and viscous-dispersive (DisCoVisc, Alcon) products. The viscoadaptive Healon 5 is highly viscous and cohesive at low-flow rate phacoemulsification but, under high-shear conditions, it fractures.20 In comparison to cohesive OVDs, the viscous-dispersive DisCoVisc is considered a higher viscosity dispersive, but has a zero-shear viscosity (i.e., viscosity at rest) like more cohesive OVDs.
It's clear that creating a perfect OVD is a massive challenge. Where one rheologic trait is needed, such as cohesiveness, another opposing trait, such as low viscosity, is also desirable.
Free-radical Scavengers
Since today's OVDs minimize the contact of instruments with tissues, some believe that most endothelial cell loss is from free radical damage.21 Free radicals are thought to be produced during cataract surgery when the ultrasonic oscillations of phaco probes instigate hydrolysis of water molecules.22,23 Hydroxide radicals then react with the fatty acids of endothelial cell membranes, resulting in the formation of lipid peroxides and, consequently, oxidative stress, apoptosis of endothelial cells and corneal edema.24 This edema may be transient; however, pseudophakic corneal edema can present eight months to seven years after surgery, and is irreversible.25 The CS and HA in OVDs serve to both scavenge free radical and to lower ultrasonic vibrations;21,26 however, their ability to reduce free radical damage is related to their retention in the anterior chamber during surgery.22,27
It's the virtually seamless biocompatibility of HA and CS with the eye that allows their formulations to provide comprehensive protection during surgery. Continued research has produced hybrid OVDs that offer the best of both the cohesive and the dispersive worlds, allowing us to manipulate tissues and provide the best outcomes.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Theocharis DA, Skandalis SS, Noulas AV, et al. Hyaluronan and chondroitin sulfate proteoglucans in the supramolecular organization of the mammalian vitreous body. Connective Tissue Research 2008;49:124-8.
2. Scott JE. The chemical morphology of the vitreous. Eye 1992;6:553-5.
3. Macknight ADC, McLaughlin CW, Peart D, et al. Formation of the aqueous humor. Clin Exp Pharma Physiol 2000;27:100-6.
4. Miller D, Stegmann R. Healon: A Guide to Its Use in Ophthalmic Surgery.
5. Karlsson J, Sjögren LS, Lohmander LS. Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis: A controlled, randomized, double-blind, parallel-design multicentre study. Rheumatology 2002;41:1240-8.
6. Lamari FN. The potential of chondroitin sulfate as a therapeutic agent. Connective Tissue Research 2008;49:289-92.
7. Caron JP. Intra-articular injections for joint disease in horses. Vet Clin Equine 2005;21:559-73.
8. Simánek V, Kren V, Ulrichová J, Gallo J. The efficacy of glucosamine and chondroitin sulfate in the treatment of osteoarthritis: Are these saccharides drugs or nutraceuticals? Biomed Papers 2005;149:1:51-6.
9. Reichenbach S, Sterchi R, Scherer M, et al. Meta-analysis: Chondroitin for osteoarthritis of the knee or hip. Ann Intern Med 2007;146:580-90.
10. Bishop PN. Structural macromolecules and supramolecular organization of the vitreous gel. Prog Ret Eye Res 2000;19:3:323-44.
11.Davidson EA, Meyer K. Chondroitin, a new mucopolysaccharide. Journal Biol Chem 1954;211:2:605-11.
12. Yanagishita M. A brief history of proteoglycans. Experientia 1993;49:366.
13. Harrison SE, Soll DB, Shayegan M, Clinch T. Chondroitin sulfate: A new and effective protective agent for intraocular lens insertion. Ophthalmology 1982;89:1254.
14. Miller D, O'Connor. Use of Na-Hyaluronate during intraocular lens implantation in rabbits. Ophthalmic Surg. 1977;8:6:58-61.
15. Arshinoff SA. Using BSS with viscoadaptives in the ultimate soft shell technique. J Cataract Refract Surg 2002;28:1509-1514.
16. Liesgang TJ. Viscoelastic substances in ophthalmology. Surv Ophthalmol 1990;34:4:268-93.
17. Madsen K, Stenevi U, Apple DJ, Harfstrand A. Histochemical and receptor binding studies of hyaluronic acid and hyaluronic acid binding sites on corneal endothelium. Ophthalmic Pract 1989;7:92-97.
18. Poyer JF, Chan KY, Arshinoff SA. A new method to measure the retention of viscoelastics on a rabbit corneal endothelial cell line after irrigation and aspiration. J Cat Refract Surg 1998:24:1:84-90
19. Arshinoff SA, Jafari M. New classification of ophthalmic viscosurgical devices – 2005. J Cataract Refract Surg 2005;31:2167-71.
20. Mamalis N. OVDs: Viscosurgical, viscoelastic and viscoadaptive. What does this mean? J Cataract Refract Surg 2002;28:9:1497-8.
21. Augustin AJ, Dick HB. Oxidative tissue damage after phacoemulsification: Influence of OVDs. J Cataract Refract Surg 2004;30:424-7.
22. Takahashi H, Sakamoto A, Takahashi R, et al. Free radicals in phacoemulsification and aspiration procedures. Arch Ophthalmol 2002;120:1328-52.
23. Nemet AY, Assia EI, Meyerstein D, et al. Protective effect of free-radical scavengers on corneal endothelial damage in phacoemulsification. J Cataract Refract Surg 2007;33:310-5.
24. Murano N, Ishizaki M, Sato S, et al. Corneal endothelial damage by free radicals associated with ultrasound oscillation. Arch Ophthalmol 2008;126:6:816.
25. Narayanan R, Gaster RN, Kenney MC. Pseudophakic corneal edema: A review of mechanisms and treatments. Cornea 2006;25:9:993-1004.
26. Frohn A, Dick HB, Fritzen CP, et al. Ultrasonic transmission in viscoelastic substances. J Cataract Reftact Surg 2000;26:282-6.
27. Oshika T, Okamoto F, Kaji Y, et al. Retention and removal of a new viscous dispersive ophthalmic viscosurgical device during cataract surgery in animal eyes. Br J Ophthalmol 2006;90:4:485-7.