The use of antimetabolite drugs has emerged as an important adjunct to glaucoma filtration surgery, specifically for high-risk cases. Trabeculectomy is designed to lower intraocular pressure by allowing the passage of aqueous humor from the anterior chamber to the subconjunctival space. The main source of failure for filtering surgery results from fibroblast proliferation and subsequent scar formation at the surgical site.
In the early 1980s researchers started to look for ways to improve the success of filtering surgery by specifically targeting the inhibition of scar formation. Among the different drugs that were tested, mitomycin C and 5-fluorouracil are the ones that we use most frequently today.
Indications for Antifibrotics
MMC and 5-FU are typically used in eyes at high risk for bleb failure. This includes neovascular glaucoma, uveitic glaucoma, previous failed filtering surgery, aphakia/pseudophakia, youth, African-American race, and combined glaucoma cataract procedures. These patient groups typically have a greater wound healing response leading to excessive scarring.
However, the jury still seems to be out on whether 5-FU or MMC should be used in primary trabeculectomies. One study found significant lowering of IOP with a very low complication rate with either 5-FU or MMC. Follow-up was limited, however, to one year and the participating surgeons were all experienced, fellowship-trained, glaucoma specialists. Another study found trabeculectomy with MMC to have significantly lower IOP, but also had a significant number of complications. This study had five-year follow-up data of 123 eyes treated with MMC. Some of the complications included: delayed onset of hypotony (IOP<6 mmHg) in 42 percent of eyes; bleb leaks in 14.5 percent; blebitis in 5.7 percent; and endophthalmitis in 0.8 percent.
5-FU
5-FU is a pyrimidine analogue that acts by selectively inhibiting DNA synthesis in the S and the G2 phases of the cell cycle. This allows only those cells in the synthesis phase to be affected, and the remaining cells to continue proliferation after the exposure to 5-FU is over.
5-FU was originally designed to be administered postoperatively. The Fluorouracil Filtering Surgery Study (FFSS) was a multicenter, randomized clinical trial that compared the outcome of trabeculectomy with and without postoperative subconjunctival 5-FU injections in eyes that had undergone prior cataract surgery or failed filtering surgery. In the study, 5 mg of 5-FU was injected subconjunctivally 180 degrees away from the trabeculectomy site twice daily in the first postoperative week and once daily in the second postop week.
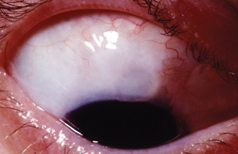 |
| A patient with blebitis illustrating the classic "white on red" appearance. A small hypopyon is present but there is an absence of vitreous cells, differentiating this from endophthalmitis. |
5-FU may also be used intraoperatively. A cellulose sponge soaked in 50 mg/ml of 5-FU is placed between the sclera and the conjunctival flap for five minutes. After removing the sponge, the eye is copiously irrigated with BSS to remove any residual 5-FU before the eye is entered. Subsequently, postoperative injections of 5-FU at a dose of 5 mg can be used in addition over the next two weeks depending on how the bleb looks. A low bleb and/or bleb vascularity can indicate impending bleb failure, and this should prompt the surgeon to consider additional 5-FU injections.
MMC
MMC is a natural alkaloid synthesized from Streptomyces caepitosus. MMC acts by reducing fibroblast collagen synthesis through inhibition of DNA-dependent RNA synthesis and has a direct cytotoxic effect. Unlike 5-FU, its action is independent of the cell cycle. It is also about 100 times more potent than 5-FU.
MMC is administered in a concentration of 0.2 to 0.5 mg/ml on a cellulose soaked sponge placed under Tenon's capsule on top of the sclera. Exposure time generally varies between two and five minutes, and is titrated depending upon the patient's potential for scarring (i.e., the greater the likelihood of scarring, the greater the length of time to use MMC.)
Complications
When deciding to use an antimetabolite with filtering surgery, it is important to weigh the risks and benefits. Keep these risks in mind when using these agents. 5-FU and MMC can cause corneal epithelial toxicity. The mechanism is thought to be the inhibition of stem cell proliferation at the limbus, which gives rise to the corneal epithelial cells. The damage can range from punctate keratopathy to large corneal abrasions. The incidence of corneal toxicity is significantly higher with 5-FU than MMC. Antimetabolites should be used with extreme caution in patients with corneal disease.
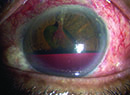
Conjunctival wound leaks also occur more frequently in patients who have been treated with MMC or 5-FU. Both antimetabolites appear to significantly decrease mitosis in conjunctival epithelium and cause thin ischemic blebs, which are more prone to late-onset bleb leaks. The thin-walled, ischemic blebs that are produced with antifibrotic use are also at greater risk to develop blebitis and bleb-related endophthalmitis.
Hypotony and hypotony maculopathy occur more frequently with the use of MMC and 5-FU. Patients who appear to be at greatest risk of this complication are young myopes. The mechanism is thought to be multifactorial, ranging from overfiltration from tissue disorganization of the bleb, decreased aqueous secretion secondary to ciliary body toxicity, with greater susceptibility in eyes with less scleral rigidity.
Patients who undergo trabeculectomies with MMC or 5-FU have a higher incidence of suprachoroidal hemorrhage. The risk factors for suprachoroidal hemorrhage include: hypertension, high preoperative intraocular pressure, advanced age, the use of anticoagulant therapy, high myopia, and prior vitrectomy.
When to Use 5-FU vs. MMC?
The greater the likelihood of failure, the more potent the antifibrotic agent that should be used. For most primary trabeculectomies, those patients at minimal risk of failure, we prefer 5-FU. We apply it intraoperatively with administration of 5-FU injections postoperatively as needed, depending on the postoperative course.
We use MMC for eyes at higher risk of bleb failure. This includes patients who have undergone previous ocular surgery, those with uveitic and neovascular glaucoma, African-American race, and young patients. The optimal concentration and length of time to apply MMC has not been determined. We use 0.4 mg/ml and titrate the length of exposure time depending upon the following model: a minimum of two minutes exposure time with the addition of one minute depending on the number of risk factors for failure (age less than 40 years, African-American race, active anterior uveitic, rubeosis iridis, previous failed filtering surgery, aphakia/pseudophakia and other previous ocular surgery) for a maximum of five minutes.
For patients at very low risk of filtering surgery failure (for example, an elderly, white patient with primary open-angle glaucoma and no previous ocular surgery), we use no antifibrotic agent.
We use 5-FU postoperatively when there is significant focal inflammation around the bleb that persists despite the use of appropriate doses of steroids. We administer the drug as a 5 mg dose in 0.1 cc subconjunctivally. The dose can be administered after focal application of proparacaine or tetracaine with a Q-tip either 180 or 90 degrees away from the bleb. Unlike mitomycin C, the risk of toxicity is low with intracameral exposure of low doses of 5-FU. Thus, the injection can be performed adjacent to the bleb in many instances.
Future Directions
What options will there be in the future? Ideally, the hope is that a medication can be developed that will selectively inhibit the scarring at the trabeculectomy site without producing complications discussed previously. As of yet, this compound has not been found, but research in this area is active.
CAT-152 (Leredelimumab), a human monoclonal antibody to Transforming Growth Factor ß2 (TGF-ß2) is currently being studied as a more physiologic agent with specific action at the trabeculectomy site. It works by reducing TGF-ß2 induced collagen production and myofibroblast-mediated contraction.
Another major area being looked at is drug delivery systems. Collagen implants, bioerodible polymers, nonbioerodible polymers, liposomes and microspheres are drug delivery systems that have been investigated to date.
Again, the goal is to deliver a precise amount of a drug, to a specific area, over a specific length of time. Currently being studied is Poly(ortho ester) (POE), a biocompatible and bioerodible polymer that was developed to deliver 5-FU in a sustained-release delivery system to decrease the amount of intraocular injections. Finally, along the lines of trying to deliver an effect at a specific treatment area, photodynamic therapy is being looked at to control scarring at the trabeculectomy site.
The intermediate and long-term complications of antimetabolite trabeculectomies, such as late-onset bleb leaks and infections, have prompted consideration of alternative surgical approaches. Glaucoma drainage implants (tube shunts) provide intraocular pressure reduction by shunting aqueous humor to an explant located in the equatorial region of the globe. A major advantage of glaucoma drainage devices appears to be a lower rate of infection; however they are associated with their own unique set of complications. Clinical situations exist in which it is unclear whether one operation is superior to the other. The Tube Versus Trabeculectomy (TVT) Study is a multicenter, randomized clinical trial that is evaluating the long-term safety and efficacy of tube shunt surgery and trabeculectomy with MMC in eyes that have undergone prior ocular surgery. In the future, the results of this study may offer some guidelines to help select the best surgical procedure for a certain group of patients.
The addition of 5-FU or MMC to trabeculectomy surgery has lead to significantly lower IOP, but it has also lead to a higher complication rate. If one plans to use an antimetabolite during surgery, it is very important to choose the patient carefully and monitor him closely for complications.
Dr. Schwartz is a clinical glaucoma fellow at the Bascom Palmer Eye Institute. Dr. Gedde is an associate professor and Residency Program director at the Bascom Palmer Eye Institute.
1. Blumenkranz MS, Ophir A, Claflin AJ, Hajek A. Fluorouracil for the treatment of massive periretinal proliferation. Am J Ophthalmol 1982;94:458-467.
2. Gressel MG, Parrish RK II, Folberg R. 5-Fluorouracil and glaucoma filtering surgery: I. An animal model. Ophthalmology 1984;91:378-383.
3. Heuer DK, Parrish RK II, Gressel MG, et al. 5-Fluorouracil and glaucoma filtering surgery. II. A pilot study. Ophthalmology 1984;91:384-393.
4. The Fluorouracil Filtering Surgery Study Group. Five-year follow-up of the fluorouracil filtering surgery study. Am J Ophthalmol 1996;121:349-366.
5. Chen CW. Enhanced intraocular pressure controlling effectiveness of trabeculectomy by local application of mitomycin-C. Trans Asia-Pacific Acad Ophthalmol 1983;9:172-177.
6. Palmer SS. Mitomycin as adjunct chemotherapy with trabeculectomy. Ophthalmology 1991;98:317-21.
7. Singh K, Mehta K, Shaikh NM, et al. Trabeculectomy with intraoperative mitomycin C versus 5-fluorouracil. Prospective randomized clinical trial. Ophthalmology 2000;107:2305-2309.
8. Stone RT, Herndon LW, Allingham RR, Shields MB. Results of trabeculectomy with 0.3 mg/ml mitomycin C titrating exposure times based on risk factors for failure. J Glaucoma 1998;7:39-44.
9. Cordeiro MF, Gay JA, Khaw PT. Human anti-transforming growth factor ß2 antibody: A new glaucoma anti-scarring agent. Invest Ophthalmol Vis Sci 1999; 40:2225-34.
10. Diestelhorst M, Grisante S. Photodynamic therapy to control fibrosis in human glaucomatous eyes after trabectulectomy: A clinical pilot study. Arch Ophthalmol 2002 Feb;120:130-4.