In recent years we’ve seen a flurry of activity in the corneal transplant arena, from front (deep anterior lamellar keratoplasty) to back (starting with Descemet’s stripping automated endothelial keratoplasty). All of these new methods have the goal of minimizing the tissue used, minimizing rejection and maximizing the speed and quality of visual recovery.
Pre-Descemet’s endothelial keratoplasty1 is a newer variant among the endothelial keratoplasty procedures, and it involves the use of Pre-Descemet’s layer (PDL) or Dua’s layer2 in the endothelial keratoplasty procedure. Though the technique is new to many surgeons, it has the potential to yield very good outcomes and may offer several advantages over previous methods. Here, we’ll explain how to get good results with PDEK.
New Layer, New Potential
In 2013, Professor Harminder Dua, MD, FRCS, chair and professor of ophthalmology at Queens Medical Centre in Nottingham, United Kingdom, and his research team demonstrated a novel pre-Descemet’s layer in the human cornea.2 Using the big bubble technique of deep anterior lamellar keratoplasty, the team injected air into the stroma of donor sclerocorneal discs and performed peeling of the Descemet’s membrane both before and after the creation of the big bubble.
They found three distinct types of big bubble: type-1, a well-circumscribed, central, dome-shaped bubble up to 8.5 mm in diameter; type-2, a large, thin-walled big bubble that always began at the periphery and enlarged centrally to form a maximum 10.5-mm diameter big bubble; and a mixed third type.
In contrast to a type-2 bubble, Descemet’s membrane could be peeled off completely without deflating the type-1 bubble. That meant there had to be an additional layer of tissue present. Furthermore, a type-1 bubble could be created after first peeling off Descemet’s membrane, confirming for the team that Descemet’s membrane wasn’t essential to the creation of a type-1 bubble.
Histologic examination of the tissue confirmed that cleavage occurred beyond the last row of keratocytes. The new layer identified by the team measured 10.15 ±3.6 µm and was composed of five to eight lamellae of predominantly type-1 collagen bundles in transverse, longitudinal and oblique arrangements.
Dr. Dua’s team suggested that this layer acts as a splint to the Descemet’s endothelium graft, providing graft stability and preventing extra scrolling of the graft tissue.1
 |
PDEK Steps
The PDEK process isn’t too different from traditional endothelial keratoplasty procedures, but it does rely on creating a good type-1 bubble at the start. Here are the steps for a successful PDEK graft:
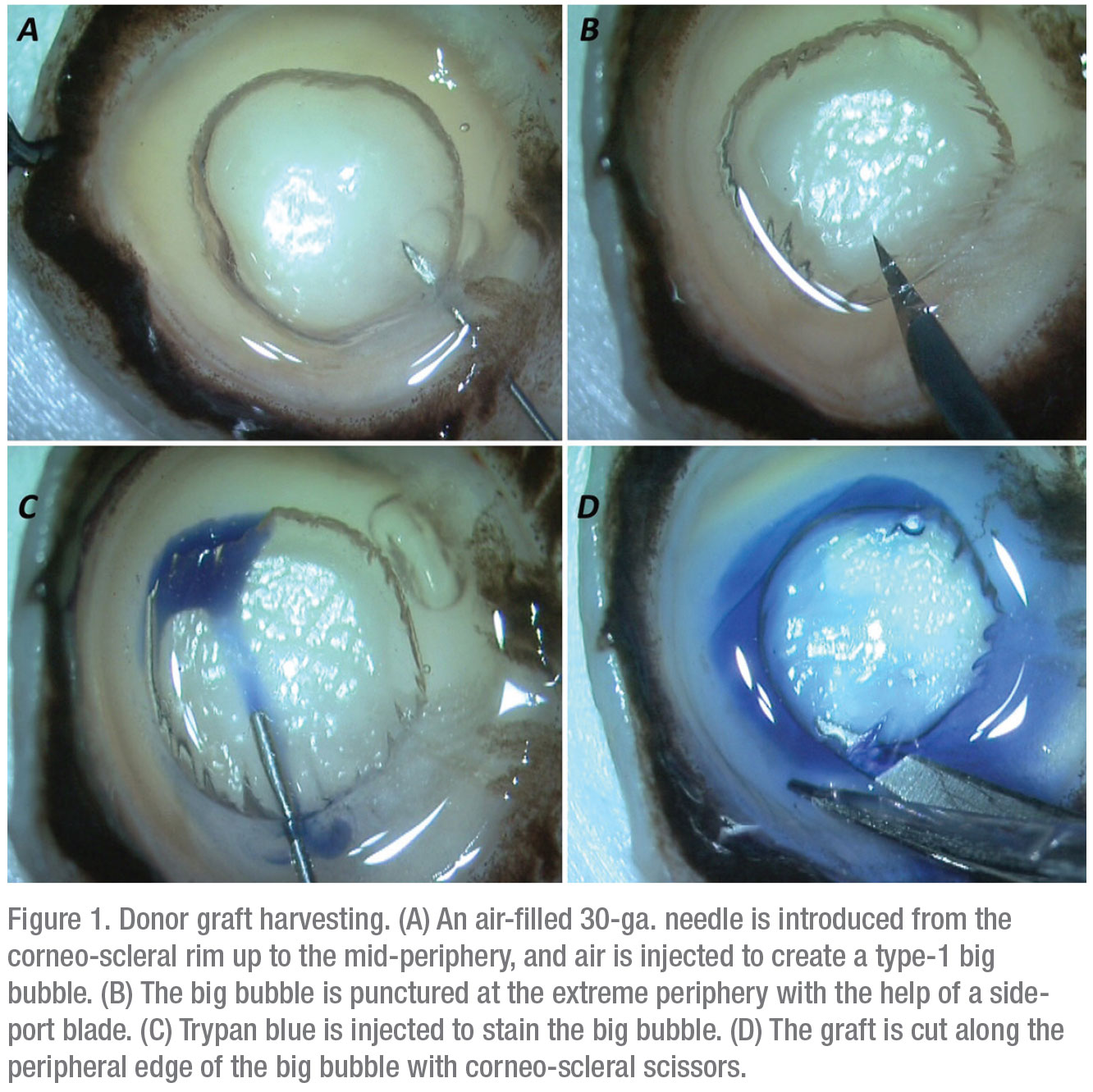
1. Donor graft harvesting. The PDEK procedure specifically depends on the formation of a type-1 bubble during donor graft preparation.2 A type-1 bubble has a distinct edge and spreads from the center to the periphery.
First, the corneo-scleral rim is placed with the endothelial side up. A 30-gauge needle attached to a 5-ml air-filled syringe is introduced from the rim into the mid-periphery of the corneal stroma. Air is then injected, which leads to the formation of a type-1 bubble. The edge of the bubble is punctured with a side-port blade and trypan blue is injected inside the bubble to stain the graft. The graft is then cut all around and harvested (Figure 1).
2. Recipient bed preparation. The recipient bed preparation is essentially the same as in other EK procedures. In cases with long-standing edema, the epithelium is usually debrided to enhance intraocular visibility of the tissues. To prepare the recipient bed, a paracentesis is made, and air is injected inside the anterior chamber. Next, descemetorhexis is performed with a reverse Sinskey hook, and the diseased DM-endothelium-complex is stripped and removed.
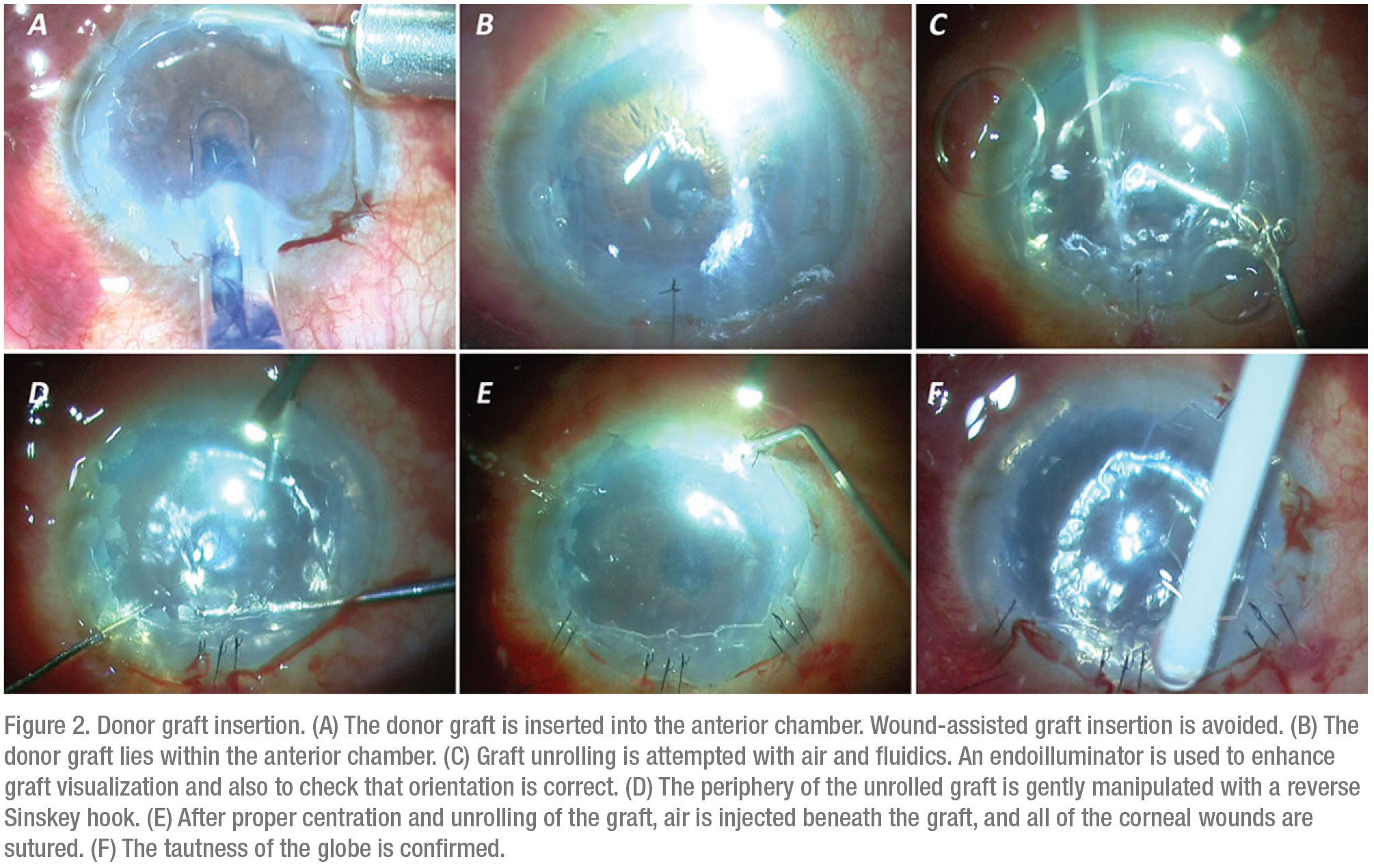
3. Donor graft injection. To inject the harvested donor graft, the graft is loaded onto the cartridge of a foldable intraocular lens where the spring of the injector system has been removed.3 The graft is then injected inside the anterior chamber. Using air and fluidics, the donor lenticule is unrolled. The surgeon must ensure that the graft is properly oriented before injecting air beneath the graft—this will ensure graft adherence to the recipient bed inside the anterior chamber.
Seal the corneal incision with a 10-0 suture. The anterior chamber should be filled completely with air to provide adequate tamponade to facilitate graft adherence (Figure 2).
4. Postoperative care and management. Immediate postoperative care mandates that the patient lie flat for at least an hour. We prescribe a topical combination of antibiotic and steroid, tapering the dose over a period of two months. At every follow-up, anterior segment OCT evaluation is used to assess the graft positioning.
 |
Techniques for PDEK
Now that we’ve gone over the steps for performing a PDEK, here are some techniques you may find useful to get the most out of the procedure:
• Endoilluminator-assisted PDEK. Patients undergoing EK procedures often have hazy corneas, which makes it difficult to assess the correct orientation of the donor lenticule. Proper graft orientation must be maintained. Under such circumstances, an endoilluminator can be used to project light obliquely onto the corneal surface. This provides transillumination that makes it easier to see the donor lenticule.4 The PDEK lenticule rolls out with the endothelial side on the outer side of the roll, as seen in DMEK.
• Combined procedures. Cases of endothelial decompensation as a sequel to IOL subluxation and decentration often necessitate a combined procedure that can be performed in one or two stages; this clearly depends on the surgical scenario, as well as the surgeon’s preference. Usually the IOL-fixation-based procedures involve either an IOL explantation or a refixation of the same IOL. Again, this depends on the surgical scenario, as well as the treatment procedure adopted for the case.
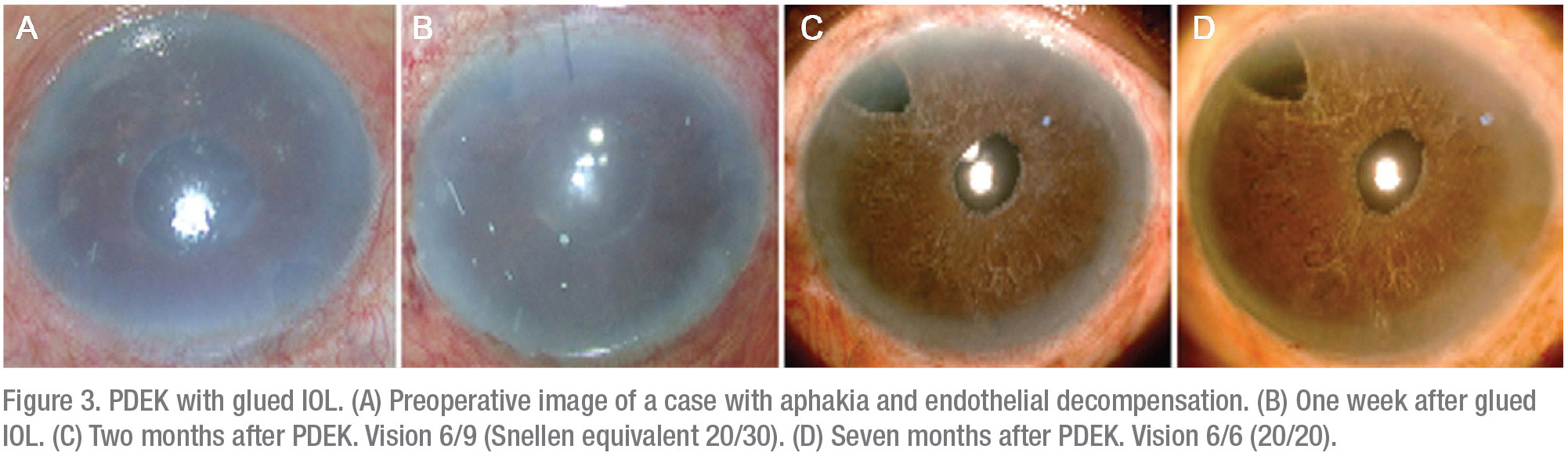
• PDEK with glued IOL. Here, the primary combination procedure involves IOL fixation/re-fixation with the glued IOL technique (Figure 3).5,6 We prefer to perform a single-stage procedure. In this approach, begin with a glued IOL and follow this with a pupilloplasty procedure.
Pupil reconstruction in these cases gives you several advantages. Often, the pupil is distorted due to vitreous prolapse; that eventually leads to endothelial decompensation. Pupilloplasty narrows the pupillary aperture and along with the IOL, acts as a barrier that helps to compartmentalize the eye efficiently into the anterior and posterior chambers. Pupilloplasty also prevents the escape of air into the vitreous cavity, facilitating the air tamponade and promoting graft adherence.
• Double-infusion cannula technique. The double infusion cannula technique (DICT) involves placing a double-infusion cannula inside the eye for a combined procedure of PDEK with glued IOL.7 To facilitate the vitrectomy procedure and to prevent the collapse of the eye, we use posterior chamber fluid infusion. Air infusion in the anterior chamber makes up the second infusion to facilitate the PDEK procedure.
Vitrectomy associated with a secondary IOL implantation leads to decompression of the posterior chamber, but this is nullified by continuous fluid infusion inside the eye. Continuous fluid infusion in a PDEK procedure prevents tonicity of the eye. A continuous seepage of fluid inside the anterior chamber helps prevent the deepening of the chamber post-vitrectomy.
• PDEK clamp. Dr. Dua and his team designed a PDEK clamp that enables the appropriate handling of donor sclero-corneal discs for achieving a type-1 bubble with appropriate air injection into the corneal stroma. The clamp has a side port for the insertion of the needle, which can be attached to an air-filled syringe. The clamp has two 9-mm diameter rings that prevent air from escaping and that facilitate the formation of a type-1 bubble by closing the fenestrations in the periphery of the PDL, thus avoiding the formation of a type-2 bubble.
• Type-2 bubble trouble. Occasionally, a type-2 bubble is formed instead of a type-1 bubble. The clinical situation then requires DMEK to be performed instead of a PDEK procedure. Pre-cut PDEK tissue is currently available to help the surgeon overcome the uncertainty of graft preparation and potential monetary loss.
 |
 |
Where PDEK Fits in EK
The visual outcomes of UT-DSAEK and DMEK have been found to be comparable throughout the entire follow-up period, and better than DSAEK outcomes, in terms of final 20/20 visual acuity and speed of visual recovery.9 This suggests that thicker donor tissue leads to greater interface haze and other refractive problems.
The spectral domain-OCT in vivo analysis of PDEK grafts has shown mean graft thickness at one-month follow-up to be 28 ±5.6 µm. This value is less than the thickness of donor tissue with UT-DSAEK (≤100 µm).10 The overall graft diameter of the PDEK lenticule varies from 7 to 8 mm, and is comparatively smaller than DMEK tissue.
Usually a donor age group older than 40 years is preferred for any EK procedure since it leads to less curling and rolling of the donor lenticule. In PDEK, peer studies have demonstrated the successful use of infant and young donor corneas too.11 We surmise that this is due to the splinting effect of Pre-Descemet’s layer on the lenticule, which eventually leads to less rolling/curling of the donor graft. PDL has been found to be sturdy enough to maintain a stable anterior chamber during cataract surgery in combined procedures with DALK.12
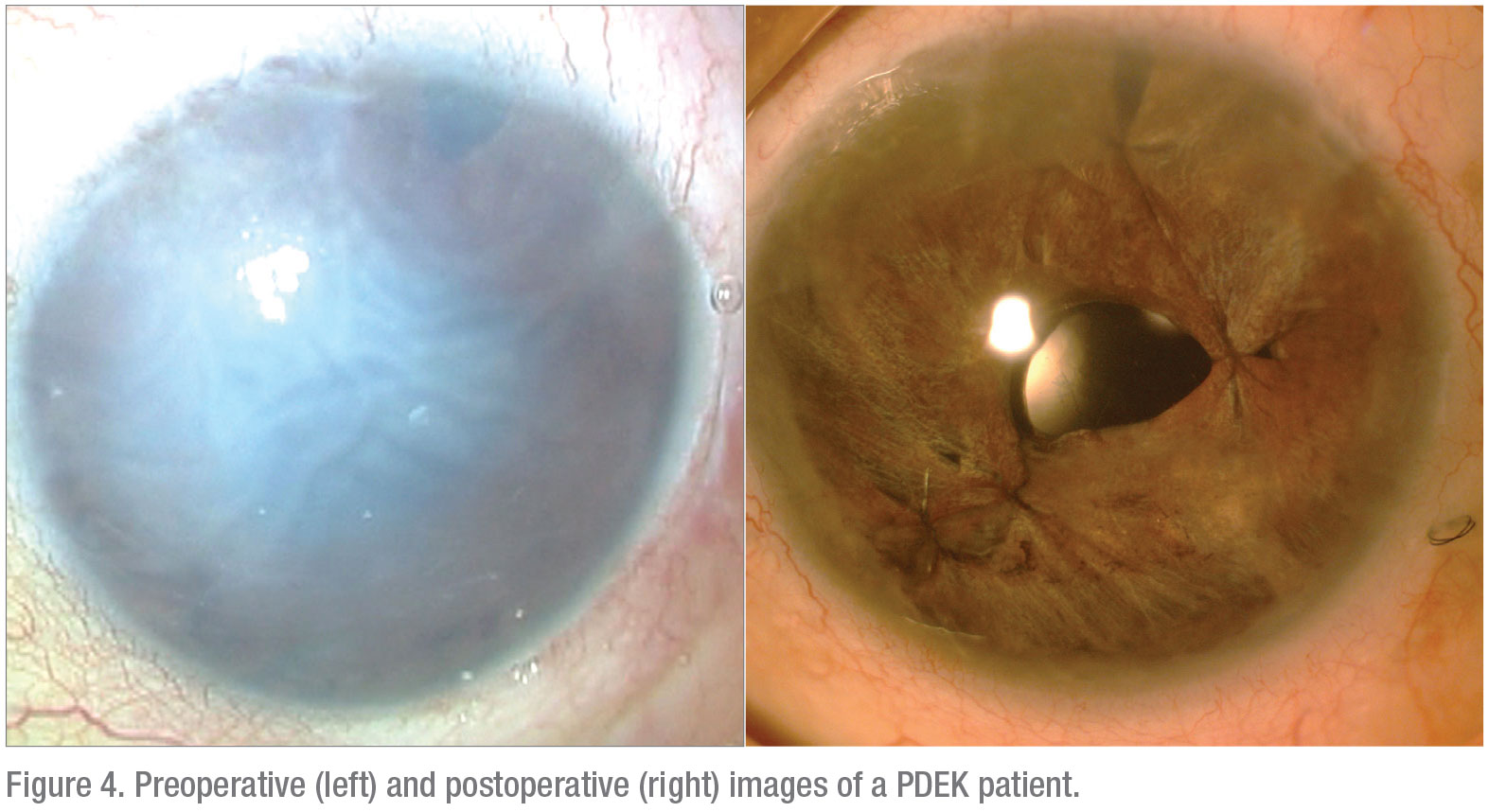
In summary, PDEK allows the employment of young donor tissue that’s usually not possible with other variants of EK procedures. In addition, PDEK serves as an effective method to achieve good visual rehabilitation in patients with a compromised endothelium (Figure 4). REVIEW
Dr. Narang is the director of Narang Eye Care & Laser Centre, Ahmedabad, India. Dr. Agarwal is a professor and head of Dr. Agarwal’s Eye Hospital and Eye Research Centre in Chennai, India. Neither has financial interest in any of the products mentioned.
1. Agarwal A, Dua HS, Narang P, et al. Pre-Descemet’s endothelial keratoplasty (PDEK). Br J Ophthalmol 2014;98:1181-1185.
2. Dua HS, Faraj LA, Said DG, et al. Human corneal anatomy redefined. A novel pre-Descemet’s layer (Dua’s layer). Ophthalmol 2013;120:1778–1785.
3. Price FW Jr, Price MO. Descemet’s stripping with endothelial keratoplasty in 200 eyes: Early challenges and technique to enhance donor adherence. J Cataract Refract Surg 2006;32:411–18.
4. Jacob S, Agarwal A, Agarwal A, et al. Endoilluminator-assisted transcorneal illumination for Descemet membrane endothelial keratoplasty: Enhanced intraoperative visualization of the graft in corneal decompensation secondary to pseudophakic bullous keratopathy. J Cataract Refract Surg 2014;40:1332–1336.
5. Agarwal A, Kumar DA, Jacob S, et al. Fibrin glue-assisted sutureless posterior chamber intraocular lens implantation in eyes with deficient posterior capsules. J Cataract Refract Surg 2008;34:1433–1438.
6. Narang P, Agarwal A, Dua HS, et al. Glued intrascleral fixation of intraocular lens with pupilloplasty and pre-Descemets endothelial keratoplasty: A triple procedure. Cornea 2015;34:1627-1631.
7. Narang P, Agarwal A. Double-infusion cannula technique for glued fixation of intraocular lens with endothelial keratoplasty. Can J Ophthalmol 2018;53:5:503–509.
8. Dua HS, Said DG. Pre-Descemets endothelial keratoplasty: The PDEK clamp for successful PDEK. Eye (Lond) 2017;31:7:1106-1110.
9. Busin M, Madi S, Santorum P, et al. Ultrathin Descemet’s stripping automated endothelial keratoplasty with the microkeratome double-pass technique: Two year outcomes. Ophthalmol 2013;120:1186–94.
10. Cheung AY, Hou JH, Bedard P, et al. Technique for preparing ultrathin and nanothin Descemet stripping automated endothelial keratoplasty tissue. Cornea 2018;37:5:661-6.
11. Agarwal A, et al. Pre-Descemet endothelial keratoplasty with infant donor corneas: A prospective analysis. Cornea 2015;34:859-65.
12. Zaki AA, Elalfy MS, Dua HS, et al. Deep anterior lamellar keratoplasty—triple procedure: A useful clinical application of the pre-Descemet’s layer (Dua’s Layer). Eye (Lond) 2015;29:3:323-6.