There’s no question that having FDA approval means a drug has been thoroughly tested for safety and efficacy. However, not having approval for a particular drug or device—or a specific use of an approved drug or device—doesn’t mean the drug or device isn’t safe and effective. That’s because the approval process itself comes with numerous real-world caveats, including:
• Executing the trials necessary to get FDA approval can be very costly. This makes it difficult or impossible for many smaller manufacturers to get approval for a product.
• Some treatments are so inexpensive that the high cost of the approval process would never be recouped. This leaves many highly effective treatments unapproved.
• Running a clinical trial may not be feasible. The size or nature of an afflicted population can make a clinical trial logistically impossible.
• Even when FDA approval is granted, it’s very specific and limited. Approval is based on the nature and outcomes of the clinical trials that led to approval. Because of cost and logistical challenges, an FDA clinical trial can’t represent the full spectrum of uses a drug may offer, and the approval will reflect that.
• The beneficial uses of a drug or device always evolve over time. If every newly discovered use of a drug or device had to go through an expensive approval process, the practice of medicine might never move forward.
In reality, many treatments that have not gone through the FDA-approval process have been well-demonstrated to be helpful and are widely used; quite a few are even standard of care. Many have been tested in clinical trials reported in the peer-reviewed literature that were not done under FDA supervision. Others that are less amenable to testing in clinical trials have been shown to be safe and effective over the course of many years of use in the real world.
The Compounding Factor
Compounding drugs into different formats or combinations has been common practice for many years, allowing doctors to address conditions beyond those specifically approved by the FDA. Despite this, obtaining many compounded drugs has recently become far more difficult as a result of the Drug Quality and Security Act. The
 |
| As a result of recent regulations, many compounding pharmacies that once provided scores of compounded drugs now compound fewer than a dozen. |
As it turns out, these well-intended new rules, meant to increase safety, are apparently having an unintended side effect: curtailing the availability of eyesight-saving medications. The cost of implementing new procedures, such as extensive safety testing, has caused many compounding pharmacies to drastically curtail their offerings. For example, in 2013, prior to the implementation of the DQSA, one pharmacy that’s still in operation offered a list of almost 200 compounded drugs and injectables. That list included five anti-allergy preparations; 38 antibiotic preparations; seven antivirals; 10 antifungals; three cytotoxic agents; nine preparations used for diagnosis (e.g. lissamine green); 26 compounds for treating dry eye; 32 preparations for managing glaucoma; and more than 60 other compounded medications. Today, as a result of the DQSA, that pharmacy has converted to a 503B outsourcing facility and offers a total of 10 compounded formulations.
The new rules are also causing other unintended consequences. Among those is a new definition of compounding that impacts some common, beneficial surgical practices. For example, adding epinephrine to the lidocaine used to numb the eye for cataract surgery is a common surgical practice. It’s supported by many peer-reviewed studies, and it demonstrably makes the surgery easier, safer and less costly by keeping the pupil dilated during the course of the surgery. The new guidelines regarding compounding encompass many common practices like this, making these practices a violation of the rules.
Here, three surgeons discuss their experiences obtaining and using off-label medications and how the current situation has affected their practices.
Going Off-label
Natalie A. Afshari, MD, FACS, Stuart I. Brown MD Chair in Ophthalmology in Memory of Donald P. Shiley; professor of ophthalmology; and chief of cornea & refractive surgery at the Shiley Eye Institute, University of California San Diego, is the chair of the FDA Committee for the American Society of Cataract and Refractive Surgery. Dr. Afshari notes that there are many scenarios in which ophthalmologists use drugs that are off-label or have to be compounded. “These drugs are very important in practice for many ophthalmology subspecialties,” she says. “Retina specialists mainly use Avastin and antibiotics; those of us treating the cornea use antibiotics and anti-amoeba medications; glaucoma specialists use mitomycin-C; and cataract surgeons use intracameral injections of antibiotics.
“An acanthamoeba corneal ulcer can be devastating to vision,” she continues. “There’s no approved drug for the treatment of acanthamoeba, so we use options such as polyhexamethylene biguanide—a pool cleaner that can be diluted—to kill the amoeba. This compounded eye drop should be inexpensive, but over time it’s become very costly and is no longer easily accessible. We recently treated a patient with an acanthamoeba corneal ulcer, and unsuccessfully spent two hours calling every compounding pharmacy we knew of to locate these drops. I ended up texting my colleagues around the country to find out where I could find these drops, and we eventually found a pharmacy many hours away that still prepares it. Another option for treating acanthamoebic keratitis is chlorohexidine, but physicians encounter similar difficulties obtaining compounded chlorohexidine.”
Dr. Afshari notes that there are other kinds of corneal ulcers that require compounded drugs for treatment as well. “We may treat bacterial ulcers with compounded antibiotics such as vancomycin or tobramycin,” she says. “Unfortunately, there are fewer and fewer compounding pharmacies in the U.S., which affects the availability of these medications. Another compounded medicine is topical alpha interferon for the treatment of ocular surface neoplasia. (The alternative for these patients is surgical excision.) Decreasing availability of topical interferon has made it difficult for some patients with ocular surface neoplasia to pursue this less-invasive therapy. Similarly, tacrolimus is a compounded medicine that is used to address recalcitrant atopy in patients with allergic eye disease. Although it’s still available in the United States, tacrolimus has become increasingly difficult to find. We’ve called many different pharmacies before finding one that prepares tacrolimus.”
 |
It’s important to note that while some of the nonapproved treatments involve drugs that were approved for other medical purposes, some drugs haven’t been approved by the FDA at all. “There is a difference,” says Dr. Afshari. “For example, the diluted pool cleaner we use to treat acanthamoeba, polyhexamethylene biguanide, isn’t approved. What company would spend millions of dollars to get approval for a pool cleaner? It wouldn’t make economic sense for them to do that. Nevertheless, for years it’s been the practice to use this chemical for treatment of Acanthamoeba. We even published a paper showing that it works in Aspergillus keratitis.1 It hasn’t been approved, so it’s considered off-label. But it is standard of care.”
Compounding Onsite
One side effect of the cutback in available compounded medications is that practices have been forced to do more drug compounding on their own in order to save some patients from vision loss or blindness.
Richard Duffey, MD, a corneal specialist in Mobile, Ala., who also teaches at the University of South Alabama, notes that recent regulations have made it extremely expensive for pharmacies to continue offering these services. “In addition to the cost of the equipment, the rare cases in which something has gone wrong have raised the cost of insurance to the point at which pharmacies can’t cover their costs,” he says. “This became an issue of liability in Alabama, so our sources stopped compounding sterile eye drops for us. Now we have to go out of state to find a place that has these commercially available, with the delay that entails. In some cases, the compounded drops are not available anymore, anywhere. Then we have to make them up ourselves.”
Dr. Duffey says his practice has to create drops for patients at least a couple of times a month—more than 25 times a year. “If you’re a corneal specialist, I don’t see how you can treat people without having to go outside of the box,” he says. “Sometimes we have to treat a problem the way we treated it in the past, despite the fact that we can’t get pharmacies to compound these medications locally. We need them to be readily available. The alternative is letting the patient lose an eye.”
Dr. Duffey says a similar problem arises with antifungals. “We often treat local peanut farmers who spray antifungals onto their peanut crops,” he explains. “They can get a black fungus called Aspergillus that’s very resistant to treatment. One young patient had a very deep and extensive
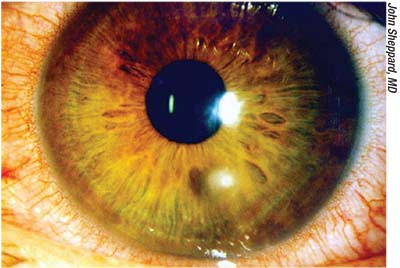 |
| Corneal problems such as ulcers frequently require treatment with compounded medications; lack of timely availability may put the eye at risk. |
“In the case of this patient, we used this method to make up a fortified antifungal solution at a concentration that allowed us to inject it directly into his cornea to treat the infection,” he continues. “We also created sterile eye drops for the patient to use. This patient ended up with a very good result after several months of treatment, although the infection recurred about three weeks after discontinuing the medication. We had to go through a second round of treatment before we completely eradicated it, which is not unusual with a fungal infection. They tend to be deep and hard to eradicate even after treating for several months.”
Dr. Duffey notes that some medications are FDA-approved to treat infections—just not specifically in the eye. “The guidelines don’t necessarily make a distinction as to where that infection is,” he says. “In this case, the problem was to get it into an eye-drop form, because an IV medication is no help when a patient has a deep corneal ulcer. We need to be able to put the medication where the fungal infection is via an injection into the cornea or a sterile eye drop.”
Dr. Duffey mentions another treatment that his practice has to create in-house: acetylcysteine eye drops. “A number of my patients use the acetylcysteine drops we make up,” he says. “The drops are made by diluting the product Mucomyst, which is prescribed to break up pulmonary secretions—thick mucous in the lungs. Pulmonary doctors often prescribe this drug in a nebulizer, allowing the patient to breathe it in. Patients with severe dry eye often develop filamentary keratitis, mucous filaments on the cornea; this medication, when diluted, does a fabulous job of dissolving those filaments. That makes it a very important medication from a patient comfort and visual acuity standpoint, especially for someone who gets these filaments recurrently. Acetylcysteine is not commercially available as an eye drop, so we simply make up the drops in the office.
“When we have to create drops in our practice, we use sterile technique in a specially cleaned space under sterile conditions, and to my knowledge, we’ve never caused a problem in any patient,” Dr. Duffey adds. “On the other hand, if we don’t make up these medications, some of our patients are going to lose an eye to an infection.”
Dr. Duffey acknowledges that compounding a drug in-practice does mean accepting liability if something goes wrong. “Is it worth taking on the liability when an eye will be significantly damaged without this treatment? In my opinion it is,” he says. “I’ve been in practice almost 30 years since my fellowship, and many of these are treatments we routinely used 30 years ago. I can’t conceive of going backwards in medicine. Would I rather take on some increased liability or watch some of my patients lose an eye, knowing that five years ago I could have treated their problem? You do what you need to do to save your patients’ vision.”
Speed of Access
Dr. Afshari points out that being able to obtain a medicine within a few hours is often a big issue. “Compounding pharmacies are good about getting the drug to you or the patient quickly,” she says. “Once we order it, they’ll FedEx it overnight so the patient gets it by the next day. However, in the case of an infection, it’s preferable to not wait even that long, because many infections progress significantly in 24 hours. In some situations it would be helpful if in-house pharmacists could make up small quantities of a drug such as vancomycin or tobramycin for the patient, to avoid delaying time-sensitive treatment.”
Dr. Duffey says he frequently needs to treat local patients with fortified antibiotics or antifungals that are not readily available. “Central corneal ulcers that are bacterial in nature need treatment with fortified antibiotics,” he points out. “Traditionally, pharmacies would compound them for us, but today, at least here in Alabama, it’s almost impossible to find a compounding pharmacy that will make sterile eye drops. And it’s very hard to get them in a timely fashion when you have to order them from out of state, which can involve a 24- to 48-hour delay.”
Some highly beneficial drugs are not even sold in the United States, although they may be easily obtained outside the country. “I sometimes have drugs mailed to me by pharmacies in Australia,” says Dr. Duffey. “A drug like Brolene [propamidine isetionate], an antiparasitic for Acanthamoeba infections, is available at local apothecary or pharmacy shops in England for the equivalent of about $10 or $12. So, whenever I go to England I buy half a dozen bottles, bring them back and keep them in my refrigerator. I use them whenever I need to treat an Acanthamoeba infection, as a supplement to other stronger pharmacy-formulated medications that are available outside the state. Luckily, Acanthamoeba is slow-growing, so the patient won’t be seriously harmed if obtaining the treatment takes several days.”
Dr. Afshari occasionally treats acanthamoebic keratitis with Brolene as well. “Sometimes our patients find out about this medication online and obtain Brolene on their own from the United Kingdom, and then send it to themselves via a family member,” she says.
Dr. Afshari says that serum tears are another treatment that’s becoming more and more difficult to obtain. “These tears are created from the patient’s blood,” she explains. “They can be very helpful for healing persistent corneal epithelial defects, but fewer and fewer pharmacies are making them. Our practice is in California, but we now have to send a patient’s blood to Kansas to get the serum tears made, which delays treatment and increases cost.”
“In many practices, it used to be that if you needed serum tears you’d simply draw the blood and send a technician to centrifuge it and dilute it down,” Dr. Afshari continues. “Physicians did this because it would allow them to provide the tears to the patient right away. Eventually, however, they stopped doing it themselves because of regulations. Similarly, fortified vancomycin and tobramycin are still available through compounding pharmacies, but I know of practices that were making their own vancomycin drops and giving them to the patient, because patients were unable to afford it otherwise.”
Expertise Counts
As noted, there’s always some risk associated with using drugs in an off-label manner, but Dr. Duffey points out that a doctor is less likely to run into trouble using off-label options if it’s clear that the alternatives have been exhausted. “As a corneal specialist, it’s pretty easy for me to justify making up these formulations because I know I’ve exhausted all the other options,” he says. “If you’re not a corneal specialist, people could say, ‘Why didn’t you go to a corneal specialist or a fellowship-trained person who was absolutely sure of the diagnosis and treatment alternatives?’
“If you’re not an authority in the area in question, I’d advise finding a good specialist and deferring to his or her judgment about what the treatment options are,” he concludes. “When your patient’s treatment needs fall outside of what you can treat with the medications that are available, hopefully that doctor will know of other alternatives.” REVIEW
Drs. Chang, Afshari and Duffey have no financial interest in any subject or product mentioned. For more information regarding the pharmacies they use, you can contact Dr. Duffey at richardduffey@gmail.com, or Dr. Afshari at naafshari@ucsd.edu.
1. Rebong RA, Santaella RM, Goldhagen BE, et al. Polyhexamethylene biguanide and calcineurin inhibitors as novel antifungal treatments for aspergillus keratitis. Invest Ophthalmol Vis Sci 2011;52:10:7309-15.
2. Chang DF, Braga-Mele R, Henderson BA, Mamalis N, Vasavada A. Antibiotic prophylaxis of postoperative endophthalmitis following cataract surgery: Results of the 2014 ASCRS Member Survey. J Cataract Refract Surg 2015;41;1300-1305.



