Trifocal IOLs
In an effort to improve intermediate vision, trifocal IOLs rely on multiple foci to achieve this range of vision. With bifocal IOLs focusing mainly on near and distance visual acuity, trifocal IOLs, in use outside of the United States, also target intermediate vision. Trifocal IOLs have two repeating steps (intermediate and near), spread over the surface of the lens. This pattern allows for a flat area between steps to redirect light for distance.
 |
| The Zeiss AT-LISA trifocal intraocular lens has an intermediate visual distance target of 80 cm. The lens is trifocal in the center and bifocal in the periphery. |
The three mainstream trifocals are the Alcon PanOptix, FineVision PhysIOL and the Zeiss AT-LISA. These lenses differ in a few ways, including how they distribute light: The Zeiss and PhysIOL use 33 percent distance, 33 percent intermediate and 33 percent near. The PanOptix distributes 50 percent of the light for distance and 25 percent each for intermediate and near vision. The lenses also differ in the location of their focal points. Zeiss and PhysIOL have an intermediate vision target of 80 cm, while PanOptix’s target is 60 cm.
They also differ in design. The Zeiss lens is trifocal in the center and bifocal in the periphery. The FineVision lens is apodized over the entire surface, so there is less light transferred to the intermediate points at the periphery. This allows enhancement of far vision the wider the pupil becomes. Although these lenses are only available overseas, a new wave of studies and their results might soon push these lenses into the U.S. market.
LensGen IOL
This clinical-stage lens uses the eye’s natural accommodative mechanisms to change the IOL’s curvature and the eye’s range of vision from distance to near.
The design starts with a fixed lens, similar to a standard IOL. There is also a fluid lens, which sits inside the fixed lens to accommodate at different distances based on the lens’s curvature. When the ciliary muscle contracts, zonules stretch, putting pressure on the capsule, which results in the change of shape. For this process of accommodation, with the lens implanted, the lens transfers the force to the fluid lens, letting it change shape in a way similar to the crystalline lens. Because it mimics the natural lens, the company says there’s a seamless range of vision from far to near.
Another benefit of this IOL is that it can be implanted during cataract surgery in two steps, which involve first inserting the fixed lens and then injecting the fluid lens. Ostensibly, this two-part implantation system would solve the challenge of implanting a large lens through a small incision.
The lens conforms to the natural shape and volume of the capsule, in the hope of preserving the integrity, function and stability of the lens so that it’s comparable in function to the natural lens. LensGen also claims that the lens is a complete vision-correction platform: The fixed lens treats myopia, hyperopia and astigmatism, while the fluid lens treats presbyopia by providing dynamic range of vision.
Uday Devgan, MD, discusses the timetable for this clinical stage lens. “It’s promising enough that we just got $21 million of funding,” he says. “A lot of these ophthalmic companies are showing interest in it based on our initial results. This funding will hopefully speed up our journey to market, which, in the U.S., I’d say is somewhere in the five- to 10-year range. It’s hard to nail down, but that’s what we’re looking at now.”
AcuFocus IC-8
AcuFocus’ IC-8 is a small-aperture IOL that extends depth of focus by combining the KAMRA corneal inlay’s small-aperture technology with a monofocal lens. Using the same small-aperture optics as the KAMRA, IC-8 incorporates a non-diffractive 2.23-mm diameter opaque mask with a 1.36-mm central aperture embedded within a 6-mm, one-piece, hydrophobic acrylic lens. This mask then creates a pinhole effect, delivering nearly 3 D of extended depth of focus by blocking unfocused peripheral light and isolating more central and focused paracentral rays through the aperture. The lens can be inserted through an incision of 3.2 to 3.5 mm.
In a post-market clinical study in Europe, at the six-month follow-up, the average binocular visual acuities were 20/16 at distance, 20/20 at intermediate and 20/25 at near. AcuFocus says that visual outcomes can be further optimized by achieving refractive targets of -0.75 D in the IC-8 eye and plano in the monofocal eye. The IC-8 has received approval in select European markets but is not approved for use in the U.S.
Light-adjustable Lens
The Light-adjustable Lens from RxSight (formerly Calhoun Vision) is an intriguing technology that’s been in the pipeline for several years. It allows the surgeon to change the lens’s refractive power after implantation using a non-invasive, postoperative irradiation procedure. The LAL is a three-piece silicone lens with a diameter of
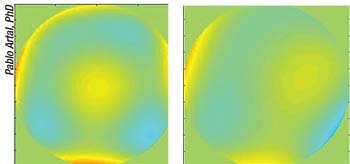 |
| Applied UV light can change the LAL’s spherical aberration, shown here pre- (left) and post- (right) irradiation. |
Once the lens is implanted, an adjustment to the lens’s structure is made by irradiating the IOL’s special silicone material with ultraviolet light, which changes the lens’s shape and therefore its power. The same light is then used to lock in the shape change when the refraction is optimal. In terms of treating presbyopia, when the ultraviolet wavelength strikes the lens in a specific pattern, it changes the distribution of the lens’s silicone macromers, resulting in an altered refractive power of the lens that yields multifocality.
The company says that trials have been positive, with the largest sample (122 eyes) showing 97 percent of patients within 0.25 D of attempted spherical equivalent and 100 percent uncorrected vision 20/25 or better.1
In February 2017, when Calhoun Vision became RxSight, the lens became known as the RxLAL. As of now, RxSight’s RxLAL is still an investigational device, not yet approved by the FDA.
FluidVision IOL
Louis D. “Skip” Nichamin, MD, a private ophthalmic surgeon and consultant in Avon, Colo., and medical advisor for FluidVision’s maker, PowerVision (Belmont, Calif.), describes this lens as the first true shape-changing lens designed to mimic the natural physiologic mechanism of accommodation. “The FluidVision technology consists of a proprietary fluid-driven system,” he explains. “Small amounts of fluid—about a drop—move in response to the natural muscle forces in the eye, changing the shape of the lens. This achieves accommodation the same way the crystalline lens does, for seamless vision from near to distance.”
According to the company, the lens was designed to be placed inside the capsular bag to protect the lens and allow conventional implantation techniques.
Dr. Nichamin says studies presented at the 2016 meeting of the European Society of Cataract and Refractive Surgeons showed excellent, stable visual outcomes, with proven accommodative function out to 36 months. “The company recently launched its new second-generation foldable lens design, the FluidVision 20/20, into the clinic,” he says. “Early results from 28 successful surgeries to date are promising, and excellent objective accommodation has been observed.”
Dr. Nichamin notes that successful implantation requires an intact capsular bag and capsulorhexis, adequate zonular integrity and essentially uncomplicated surgery. “There’s been no sign of drop-off in accommodative function over time in the patients implanted thus far,” he says. “The company is currently conducting clinical trials, with the goal of having the lens available on the market within the next few years.”
Lumina IOL
The Lumina (Akkolens International, The Netherlands) is a dual-optic, hydrophilic acrylic lens that’s placed in the sulcus. Movement of the ciliary muscles causes one of the optics to slide over the other, progressively changing the refractive power of the lens and providing its accommodative effect.
“We’ve just submitted an article for publication in which we compared subjective and objective accommodation—subjective with the defocus curve, and objective with the WAM 5500 device,” says Jorge L. Alio, MD, PhD, a professor and chairman of the department of ophthalmology at Miguel Hernandez University in Alicante, Spain. “The outcomes demonstrate that two-thirds of the near vision effect is due to an increase in the power of the eye, which means real accommodation. This rules out any pseudo-accommodation induced by other mechanisms the lens might trigger, such as pupil size.” In his studies, Dr. Alio says the accommodation has ranged from 1.5 to 6 D (mean: 2.5 D). There currently is no timetable for the lens’s approval in the United States.
LiquidVision Drops
“LiquidVision [Presbyopia Therapies; Coronado, Calif.] is an eye drop with a proprietary mixture of components that produce improved near vision without sacrificing distance vision,” explains Terry Kim, MD, professor of ophthalmology at the Duke University Eye Center. “It works via the pinhole effect, but unlike some competing products, it contains no pilocarpine. Pilocarpine causes some accommodation, undercutting distance vision. In contrast, anterior segment OCT studies have shown that with LiquidVision the anterior chamber depth stays exactly the same. In some cases LiquidVision even improves distance vision so, unlike pilocarpine, it can be used binocularly.”
Dr. Kim says the drop works within about 30 minutes of application and lasts five to six hours. “It’s reversible, and you can repeat it,” he notes. “It can be used every day or as needed, and it can be complimentary to whatever else you’re doing to enhance vision, such as contact lenses or glasses. It’s totally within the patient’s control, in terms of when they get the effect.”
Dr. Kim points out that this drop could also be therapeutic for patients with corneal scars or refractive surgery complications, especially if the patient isn’t a candidate for a surgical cure. “The only negative effect so far is a dimming effect, due to pupil constriction,” he says. “However, the studies have found that this effect fades with time.”
Dr. Kim says preclinical studies produced excellent results. “The product is in Phase II-B trials now,” he says. “Preliminary results should be available in time for the meeting of the American Academy of Ophthalmology this fall. We hope the drop will be available in about two years.”
Presbia Flexivue Microlens
The 3-mm diameter Microlens is placed in a femtosecond-created corneal pocket in a patient’s non-dominant eye. The procedure usually takes less than 10 minutes and results in an improved range of vision, Presbia says. The Microlens offers powers ranging from +1.5 D to +3.5 D in 0.25-D increments. The peripheral zone of the lens contains the power to refract light from near objects onto the retina, while the central zone has no power.
Presbia PLC reports that, in one study of the inlay, subjects gained an average of five lines of uncorrected near vision in treated eyes. Approximately 82 percent of subjects achieved 20/40 or better UCDVA vision in treated eyes, and there was little to no change in binocular UCDVA vision vs. preop. Ninety-eight percent of subjects achieved 20/40 or better best-corrected distance vision in the treated eyes and there was little to no change in binocular best-corrected distance vision from preoperative values.2
With regard to approval, the company tempers expectations, saying it can’t say for sure the timetable for FDA marketing authorization.
VisAbility Micro-Inserts
VisAbility Micro-Inserts (Refocus Group; Dallas, Texas) are premised on the Schachar theory of presbyopia, which holds that continual growth of the lens crowds the cilary body until the muscles can no longer contract effectively. This scleral expansion system props up the lens via implants designed to give the ciliary muscles more room.
A docking system fixated to the sclera guides the positioning and orientation of 400-µm scleral tunnels at each quadrant of the sclera. A scleratome makes the incisions, and an interlocking two-piece PMMA implant smaller than a grain of rice goes into each tunnel.
VisAbility Micro-Inserts are commercially available in Europe. In the United States, surgeries are complete on 360 patients enrolled in a multicenter Phase III clinical trial. Early data (ClinicalTrials.gov Identifier: NCT02374671) shows that 95 percent achieved distance-corrected near visual acuity of 20/40 or better in their dominant eye at six months, gaining an average of
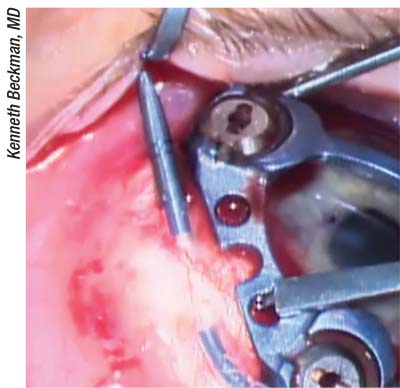 |
| VisAbility Micro-Inserts being passed through a shuttle device for insertion into scleral tunnels. |
Westerville, Ohio, surgeon Kenneth Beckman participated in the trial. “Patients ideally should be within approximately +0.50 or -0.50 of plano. While we excluded post-refractive surgery patients in the trial, they would likely be great candidates once the procedure is approved,” he says.
Although the scleral implants don’t affect the cornea and visual axis, the VisAbility procedure is not risk-free. “On the day of surgery, the main risk has to do with the location of the implants,” says Dr. Beckman. “We try to place the four segments in between the four rectus muscles. This is to make sure that the implants do not compromise the anterior segment circulation, which travels in the line of the rectus muscles. If the implants are not placed in the proper location, anterior segment circulation may be compromised. Scleral thinning and erosion over the implants are also remote possibilities.
“Long term, the main risks are dry eye and prolonged redness,” he adds. “Each of these adverse events is generally mild and treatable.”
EV06
EV06 is a prodrug (specifically, a lipoic acid choline ester, 1.5%), which penetrates the cornea and metabolizes into choline and lipoic acid. The main aim of EV06 is to soften the stiffened crystalline lens, which the company says is a major cause of presbyopia.
A Phase I/II randomized, double-masked, multicenter study examined the safety and efficacy of EV06 compared to placebo for the treatment of presbyopia. Seventy-five subjects between the ages of 45 and 55 were randomized 2:1 to receive one drop of EV06 (n=50) or placebo (n=25) b.i.d. over 90 days.
The study met both primary safety and efficacy outcomes. A significant improvement of DCNVA from baseline was observed in the EV06 group compared to placebo, with onset of DCNVA improvement beginning at day 15 (p=0.017) and continuing throughout the 90-day study period (p=0.005). EV06 outperformed placebo in objective and subjective measures throughout the study duration.4
Further steps for EV06 include a Phase IIB dose-ranging study in Q1 of 2017, followed by a Phase III pivotal study in Q2 of 2018 to closely study the long-term safety of the drug and the effects of a longer duration of treatment.
FOV Tears
Luis Felipe Vejerano, MD, Fundación Oftalmológica Vejarano, Popayan, Colombia, has developed a topical eye drop that seems to improve near vision, without compromising distance vision, for hours at a time. The formula is a combination of FDA-approved agents that he selected to provide controlled miosis without inducing ciliary muscle spasm or causing irritation to the ocular surface.
In a pilot study of 14 emmetropic presbyopes, Dr. Vejerano and colleagues administered one drop binocularly to each patient.5 Their UNVA improved by 2 to 3 Jeager lines (J 3.5 to J 1.5) without compromising UDVA. Myopic shift peaked at 0.5 D but resolved by hour four. Patients expressed satisfaction with the drops, with some saying that near visual acuity started to regress noticeably at eight hours; measurable improvement had dropped to 1 to 2 Jaeger lines at 10 hours.
Dr. Vejerano owns the patent to the drops, commercially known as FOV Tears. “The idea is to register them worldwide, including the U.S.,” says Dr. Alio, one of the study’s authors. “The best candidates for the drops have initial or intermediate presbyopia (up to 1.5 D) who wish to achieve spectacle independence for near.” REVIEW
Dr. Alio is a consultant for FOV Tears. Dr. Kim is a consultant to Presbyopia Therapies. Dr. Devgan consults for LensGen. Dr. Beckman is an investigator for the Refocus Group.
1. Hengerer F, Dick H, Conrad-Hengerer I. Clinical evaluation of an ultraviolet light adjustable intraocular lens implanted after cataract removal. Ophthalmology 2011;118:12:2382-8.
2.https://www.sec.gov/Archives/edgar/data/1591096/000156459017011561/lens-8k_20170523.htm. Accessed 8 June 2017.
3. Data on file. Refocus Group, Inc.
4. http://www.prnewswire.com/news-releases/encore-vision-announces-positive-results-from-the-phase-i-ii-study-of-topical-ev06-for-the-treatment-of-presbyopia-300260794.html accessed 12 June 2017.
5. Renna A, Vejarano LF, De la Cruz E, Alió JL. Pharmacological treatment of presbyopia by novel binocularly instilled eye drops: A pilot study. Ophthalmol Ther. 2016;5:1:63-73.



