Although soft-tissue augmentation dates back more than a century, an ideal filler has yet to be found. The most utilized non-permanent injectables in the United States have been the bovine collagens and autologous fat injections, because of accessibility and lack of any alternative substances. Soft-tissue fillers are an excellent adjunct to surgical facial rejuvenation as well as stand-alone procedures to achieve reversal of the aging process. As with any of the soft tissue fillers, one should have a familiarity with the substance to inject, technique of injecting and potential complications.
Restylane (Q-med, Uppsala, Sweden) has recently been approved by the Food and Drug Administration in the United States for the treatment of facial rhytids. It has been recommended for use in the glabellar, nasolabial and periorbital regions, and lower facial areas. Restylane is a non-animal, bacterially derived, highly crossed-link stabilized glycosaminoglycan. It is a derivative of the naturally occurring polysaccharide components of hyaluronic acid, N-acetyl-glucosamine and D-glucuronic acid. It plays a role in lubrication, stabilization and hydration of dermal cells contained within the connective tissue. Hyaluronic acid is a major constituent of the matrix surrounding collagen of the skin and is extremely hydrophilic. As the number of crossed-links increases so does the viscosity and molecular weight, resulting in a reduced absorption rate.
 |
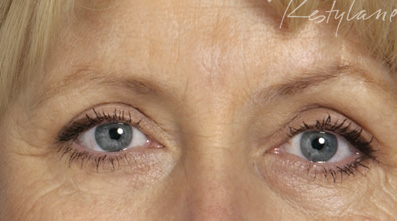 |
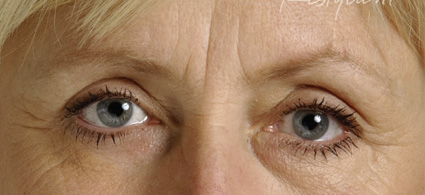
| Pre- and post-treatment with the newly approved non-animal, hyaluronic acid gel, Restylane. |
Recently Restylane has certainly grabbed its share of the hyaluronic acid spotlight even though other derivatives exist, such as Hylaform (Inamed Corp. Santa Barbara, Calif.), which is currently approved in Europe for treatment of rhytids. Restylane and the lower molecular weight derivative Perlane had been used for facial rejuvenation in countries outside the United States for several years prior to the FDA approval.
Technique
Intradermal injections are not difficult to master, though some experience with Restylane is necessary to achieve the optimal outcome. As with any other soft-tissue fillers there is a learning curve and comfort level that should be acquired before using this product. Hyaluronic acids should be deposited in the middle dermis with a 27- or 30-ga. needle with its bevel up. Prior to injection topical anesthetic cream or nerve blocks may be performed, and this is recommended when treating the perioral and lip areas.
Optimally, the needle should be at a 40 to 45 degree angle to the skin and injection should proceed directly along the rhytid to be treated. The needle should be completely inserted and, while withdrawing, the material should be deposited along the rhytid. Deposition of Restylane or Perlane in my hands results in a line of small, distinctive deposits, which are sculpted with finger pressure to achieve the desired result.
Overcorrection should be avoided since what you see is what you get in most cases of Restylane use, although one study reported satisfactory results with a 20-percent overcorrection.1 Following injection most if not all patients will develop self-limiting erythema lasting 24-48 hours. Importantly, one must remember to fully communicate to patients that this is not a permanent solution to their wrinkles, and that they will reappear over time.
Studies
Although some studies in the United States have compared Restylane to traditional fillers, most of the literature and experience is reported by our European counterparts. Several human studies found that the degree of aesthetic improvement declined steadily from the time of injection over a period of one year.2 Very few side effects were noted, but those that were included redness, swelling, lumpiness, hematoma, misplacement of the material and darkening of the injectable.2 One main advantage of Restylane versus bovine collagen is the lack of allergenicity associated with it, obviating the need for pre-treatment skin testing.
Restylane offers the clinician yet another non-permanent, dermal filling alternative for facial rejuvenation.
Dr. Cohen is a fellow at the Institute for Craniofacial and Reconstructive Surgery, Providence Hospital, Southfield, Mich. Contact him at
ajcohenmd@ prodigy.net.
1) Duranti F, Salti, G, Bovani B, et al. Injectable hyaluronic acid gel for soft tissue augmentation. Dermatol Surg 1998;24(12):1317-1325.
2) Olenius, M. The first clinical study using a new biodegradable implant for the treatment of lips, wrinkles and folds. Aesthetic Plast Surg 1998;Mar-Apr;22(2):97-101.



