The Intralase Pulsion's stint as the only femtosecond laser available for making LASIK flaps may be over, at least in Europe and Asia. Another company, 20/10 Perfect Vision (Heidelberg, Germany), maker of Visx's WaveScan aberrometer, is throwing its hat in the flapmaking ring with the Femtec laser. Though some of the principles behind the Femtec's operation are the same as the Pulsion's, there are some differences as well. Here's an early look at the new device.
The salient feature of the Femtec is where the machine meets the eye. Rather than attaching to the eye with high vacuum, the laser has a curved interface that's fixed on the cornea at low vacuum levels, so the intraocular pressure remains below 26 mmHg, sparing the patient from blacking out in the operative eye or feeling pain.
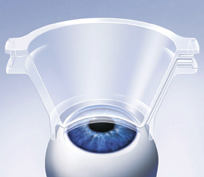 |
| The Femtec's interface is curved to match the cornea's contour. |
"The curved interface has some advantages," claims Thomas Stahr, 20/10's marketing manager. "We expect the flap to look different when cut with a curved interface that follows the cornea, rather than being cut by flattening the cornea then releasing it after ablation. With a microkeratome, you may get a flap that's thin in the middle and thicker in the periphery, but a curved interface gives an even thickness throughout the flap."
The Femtec lays down its energy in a circular pattern from the outside of the flap to the inside, and the surgeon can set parameters for depth, angle, and hinge position and width. The minimum flap thickness possible is
90 µm.
Michael Knorz, MD, of Mannheim, Germany, has used the Femtec as part of a small-scale, 50-patient study.
"The quality of the flap cut was examined by scanning electron microscopy and is excellent," he says. "It compares to the quality of a microkeratome cut. In a series of porcine eyes, the flap thickness had a standard deviation of about 5 µm. In our initial human cases we found the standard deviation to be 10 to 20 µm, but all you need to do is adjust the nomogram and then you're down to around 5-10 µm standard deviation."
Dr. Knorz says his only complications occurred in his very first cases, in which the laser made the actual cut in the epithelium, rather than deeper. "We stopped, waited a few weeks, then performed LASIK with the Amadeus microkeratome without any complications," he says.
Though the laser received U.S. Food and Drug Administration approval in February, "the United States isn't our first target," Mr. Stahr says. The company will instead begin selling the devices in Europe, then in Asia. It received a European CE Mark on April 30.
"We'll place the first three or four units in Europe in June to begin generating more clinical data," says Mr. Stahr.



