Background
The last decade has witnessed tremendous improvements in the medical and surgical management of retinal diseases, including the introduction of anti-vascular endothelial growth factor therapy, long-acting corticosteroid implants and small-gauge vitrectomy. These technologies have benefited patients tremendously, leading to a marked reduction in vision loss resulting from a broad spectrum of retinal diseases. However, despite these advances, significant vision loss still occurs, both in the acute disease setting and over time in chronic diseases such as AMD.
For example, although 95 percent of AMD patients with a new choroidal neovascular membrane maintain vision for the first two years following the initiation of anti-VEGF therapy, stability is traditionally defined as less than 15 letters lost. As such, a significant percentage of patients with wet AMD lose some vision despite the early initiation of therapy, successful resolution of macular fluid and prevention of major complications, such as submacular hemorrhage or disciform scarring. This vision loss can occur within weeks or months and is likely due to molecular pathways triggered within the retina.
|
There are equally important visual impairment concerns related to dry AMD, which is present in all wet AMD patients. Here, though, the critical pathological processes do not necessarily involve VEGF upregulation. While anti-VEGF therapy may be able to effectively limit a rapid worsening of central visual function due to wet AMD in a vast majority of treated individuals, it has little effect on the progression of retinal atrophy due to the underlying dry component. This issue is critical as dry AMD accounted for approximately 55 percent of patients with AMD in 2000, and its impact on the AMD patient landscape is growing. With dry AMD projected to affect nearly 3 million Americans by 2020, it is readily apparent that an alternative therapeutic approach is needed to address vision loss in these patients.2
Even though AMD often dominates the retinal disease discussion, there are also less prevalent conditions leading to significant vision loss for which retina specialists are seeking better therapies. An example is macula-off retinal detachment, which affects about 25,000 people in the United States each year. Surgical therapy for retinal detachment is highly effective at re-attaching the retina but it is unable to consistently prevent vision loss caused by the condition. A recent prospective trial in macula-off retinal detachment showed that the average postoperative visual acuity at one year was 0.57 logMAR or approximately 20/70.3 Clearly, a pharmacologic approach that could be combined with surgical care to improve visual outcomes in macula-off retinal detachment would provide great value to both patients and physicians. Interestingly, it may be through investigating non-neovascular retinal disease such as retinal detachment that new targets for enabling the preservation of vision, generally, are better understood.
It is widely known that photoreceptor cell death is the ultimate cause of permanent vision loss across a wide spectrum of retinal diseases. A series of studies evaluating photoreceptors in animal models of retinal detachment and cadaver eyes has enhanced our understanding of retinal and RPE cell survival and death.
|
Apoptosis & the Role of Fas in RD
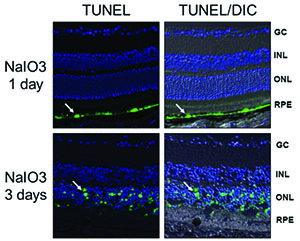
Researchers have studied the mechanism of photoreceptor cell death in retinal detachment in a rat model of the condition. Photoreceptors were found to show well-known characteristics of apoptosis such as pyknotic nuclei, TUNEL positive staining and caspase activation.4 Additional work with this model demonstrated a time-dependent formation of the FAS-receptor/FAS-ligand complex following experimental detachment, as well as upregulation of the Fas receptor and Fas ligand.5 Confirming the role of the Fas pathway in photoreceptor cell death, researchers have showed that inhibition of the Fas receptor in this model leads to photoreceptor survival in treated vs. control eyes.6-8
An important correlation to human disease was provided by a separate clinicopathologic case series in which retinal tissue fragments were excised during retinal detachment surgery and subsequently analyzed microscopically. The outer nuclear layer (location of the cell bodies of the photoreceptors) showed TUNEL positivity, leading to the conclusion that human photoreceptor cells follow a similar pattern of apoptosis to that seen in animal models.8 Taken together, this research suggests that it is likely that photoreceptor cell death due to apoptosis (and to a lesser degree, necroptosis*) is a major cause of reduced vision in macula-off retinal detachment. Inhibiting Fas-receptor activation should lead to improved photoreceptor cell survival in the clinic.
(*Necroptosis is a more organized form of necrosis in which cellular components are released into the extracellular space rather than packaged into apoptotic bodies for degradation, which occurs in apoptosis. Retinal detachment also leads to necroptosis of the photoreceptor. Activation of necroptosis, however, is downstream of the Fas-receptor. Blocking Fas-receptor activation prevents both photoreceptor apoptosis and necroptosis.26 Thus, apoptosis and necroptosis are triggered by the upstream activation of the Fas receptor by Fas ligand.)
Fas Activation in Wet AMD
The hallmark of wet AMD is the development of a choroidal neovascular membrane. CNV, in turn, leads to leakage of plasma into the subretinal space, as well as intraretinal edema. Alterations in the relationship between the photoreceptor layer and the RPE thus occur at a very physical level. Clearly, although the extent of fluid collecting under the neurosensory retina may be much greater in a bullous macula-off retinal detachment, the fundamental process is similar—a separation between the RPE and photoreceptors. And, not surprisingly, photoreceptor apoptosis has been demonstrated in a mouse model of laser-induced CNV. Further research with cadaver eyes provides clinical support for photoreceptor death by apoptosis through the demonstration of TUNEL-positive staining and Fas receptor upregulation in the macular region of human eyes with wet AMD.11 Additionally, work on both surgically excised CNV in AMD and on relevant animal models has described apoptosis in RPE cells.12,13
This information is important for several reasons. First, it confirms activation of the Fas pathway in wet AMD in humans. Second, it shows that the RPE as well as photoreceptors undergo Fas-induced apoptosis in human retinal disease. Third, the death of the RPE will inevitably lead to a separation between RPE and photoreceptors in wet AMD. Thus, a mechanism for both RPE and photoreceptor apoptotic cell death in wet AMD has been identified.
Fas Activation in Dry AMD
The role of Fas-mediated photoreceptor apoptosis also extends to the area of dry AMD, as supported by data from both animal and human studies. In animal models of retinal degeneration, including light-induced damage in rats and in mice strains with genetic mutations, the death of photoreceptors via apoptosis has been clearly demonstrated. It has been proposed that despite different species, genotypes and phenotypes, photoreceptor apoptosis is the final common pathway in these pathological processes.14-18 Additionally, more than a decade of clinical research into the underlying mechanisms of exudative AMD has delivered findings that support the likelihood that dry AMD leads to photoreceptor cell death via Fas-induced apoptosis.11,19
But what of the RPE? Even if in dry AMD photoreceptors die from a pathway amenable to intervention such as targeting the Fas receptor, is it realistic to think that vision in patients could be preserved over significant periods of time when the RPE—which provides critical metabolic support to the photoreceptor—is also dying? The answer is likely that for long-term retinal stability both functional RPE and photoreceptors are needed. If, however, RPE cells were dying in dry AMD from the same pathway as the photoreceptor, then it is possible that a therapeutic agent targeting that pathway could be beneficial to both cell types.
| ||||||||||
• Human RPE cells normally express low levels of the Fas receptor; oxidative stress regulates the expression of Fas-receptors in the RPE; and stressed human RPE shows increased Fas receptor expression.20,21
• Dry AMD, Fas and changes to the blood retinal barrier are implicated in a positive feedback loop leading to RPE apoptosis.22
• Aging in animal models is associated with increased blood concentration of soluble Fas ligand, a finding that also correlates with a risk factor for dry AMD—age.23
• In a sodium-iodate RPE toxicity model, RPE cells die by apoptosis before photoreceptors secondarily die by apoptosis. (Besirli CG, et al. IOVS 2011;52:ARVO E-Abstract 4458.)
Based on this broad collection of research, it is highly likely that vision loss in dry AMD is a Fas-dependent apoptotic process and the Fas pathway leads to cell death in both photoreceptors and RPE cells. Accordingly, the opportunity to simultaneously target RPE and photoreceptor survival via a single inhibitor of Fas-receptor activation is feasible.
Inhibitors of Fas Activation
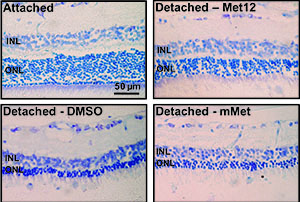
Fortunately, therapeutic agents capable of inhibiting Fas activation exist. Furthermore, these agents are amenable to ocular delivery. Approaches include a Fas-receptor-neutralizing antibody, small inhibitory RNA against the Fas receptor and a small peptide inhibitor of the receptor. Each approach has demonstrated decreased rates of apoptosis of photoreceptors following experimental retinal detachment.24,25 In particular, a small peptide inhibitor called Met12, which originated from knowledge of the met oncoprotein, has shown significant promise in this setting.
Today, as outgrowths of experimental work on Fas and apoptosis in retinal detachment, novel research is being conducted on protecting photoreceptors and the RPE in multiple models of retinal disease. Molecules identified in the laboratory may promise patients better visual outcomes in the face of retinal detachment, AMD and other retinal conditions. A clear link between Fas pathway activation and a number of blinding human retinal diseases has been successfully demonstrated, leading to the belief that Fas inhibition represents a potential breakthrough approach aimed at preserving vision in at-risk patients. ONL Therapeutics Inc., a biopharmaceutical company developing novel therapies for preserving sight in a range of retinal diseases, has recently identified a more potent analog of Met12, and is planning to initiate clinical studies in 2016. REVIEW
Dr. Kleinman is a part-time associate professor of ophthalmology at the Flaum Eye Institute at the University of Rochester where he specializes in the medical and surgical care of patients with retinal disease. He has spent more than 10 years working in retinal pharmaceutical development and currently serves as the chief medical officer at ONL Therapeutics Inc.
Dr. Zacks is a professor of ophthalmology and a clinician-scientist at the University of Michigan, Kellogg Eye Center. Over the past 15 years his research has focused on the molecular regulatory mechanisms controlling photoreceptor death or survival in retinal disease. He is a co-founder of ONL Therapeutics and serves as its chief science officer.
1. Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, et al. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP). Ophthalmology 2013;120:2292-2299.
2. The Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122:564-572. doi:10.1001/archopht.122.4. 564.
3. Mitry D, Awan MA, Borooah S, Syrogiannis A, et al. Long-term visual acuity and the duration of macular detachment: Findings from a prospective population-based study. Br J Ophthalmol 2013;97:149-52.
4. Zacks DN, Hänninen V, Pantcheva M, Ezra E, et al. Caspase activation in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci 2003;44:1262-7.
5. Zacks DN, Zheng QD, Han Y, Bakhru R, Miller JW. FAS-mediated apoptosis and its relation to intrinsic pathway activation in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci 2004;45:4563-9.
6. Zacks DN, Boehlke C, Richards AL, Zheng QD. Role of the Fas-signaling pathway in photoreceptor neuroprotection. Arch Ophthalmol 2007;125:1389-95.
7. Besirli CG, Chinskey ND, Zheng QD, Zacks DN. Inhibition of retinal detachment-induced apoptosis in photoreceptors by a small peptide inhibitor of the fas receptor. Invest Ophthalmol Vis Sci 2010;51:2177-84.
8. Zacks DN, Zheng QD, Han Y, Bakhru R, Miller JW. FAS-mediated apoptosis and its relation to intrinsic pathway activation in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci 2004;45:4563–4569.
9. Arroyo JG, Yang L, Bula D, Chen DF. Photoreceptor apoptosis in human retinal detachment. Am J Ophthalmol 2005;139:605-10.
10. Wang HC, Brown J, Alayon H, Stuck BE. Transplantation of quantum dot-labelled bone marrow-derived stem cells into the vitreous of mice with laser-induced retinal injury: Survival, integration and differentiation. Vision Res 2010;50:665-73.
11. Dunaief JL, Dentchev T, Ying GS, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol 2002;120:1435-1442.
12. Hinton DR, He S, Lopez PF. Apoptosis in surgically excised choroidal neovascular membranes in age-related macular degeneration. Arch Ophthalmol 1998;116:203-9.
13. Bhisitkul RB, Winn BJ, Lee OT, Wong J, et al. Neuroprotective effect of intravitreal triamcinolone acetonide against photoreceptor apoptosis in a rabbit model of subretinal hemorrhage. Invest Ophthalmol Vis Sci 2008;49:4071-7.
14. Chang GQ, Hao Y, Wong F. Apoptosis: Final common pathway of photoreceptor death in rd, rds, and rhodopsin mutant mice. Neuron 1993;11:595-605.
15. Portera-Cailliau C, Sung CH, Nathans J, Adler R. Apoptotic photoreceptor cell death in mouse models of retinitis pigmentosa. Proc Natl Acad Sci U S A 1994;91:974-978.
16. Reme CE, Grimm C, Hafezi F, Marti A, Wenzel A. Apoptotic cell death in retinal degenerations. Prog Retin Eye Res 1998;17:443-464.
17. Perche O, Doly M, Ranchon-Cole I. Caspase-dependent apoptosis in light-induced retinal degeneration. Invest Ophthalmol Vis Sci 2007;48:2753-2759.
18. Sanges D, Comitato A, Tammaro R, Marigo V. Apoptosis in retinal degeneration involves cross-talk between apoptosis-inducing factor (AIF) and caspase-12 and is blocked by calpain inhibitors. Proc Natl Acad Sci U S A 2006;103:17366-17371.
19. Johnson PT, Lewis GP, Talaga KC, Brown MN, Kappel PJ, Fisher SK, Anderson DH, Johnson LV. Drusen-associated degeneration in the retina. Invest Ophthalmol Vis Sci 2003;44:4481-88.
20. Esser P, Heimann K, Abts H, Fontanta A, Weller M. CD95 (Fas/APO-1) antibody-mediated apoptosis of human retinal pigment epithelial cells. Biochem Biophys Res Comm 1995;213:1026-34.
21. Lin H, Qian J, Castillo AC, Long B, Keyes KT, Chen G, Ye Y. Effect of miR-23 on oxidant-induced injury in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 2011;52:6308-14.
22. Hettich C, Wilker S, Mentlein R, Lucius R, Roider J, Klettner A. The retinal pigment epithelium (RPE) induces FasL and reduces iNOS and Cox2 in primary monocytes. Graefe’s Arch Clin Exp Ophthalmol 2014;252:1747-54.
23. Zhao H, Roychoudhury J, Doggett TA, Apte RS, Ferguson TA. Age-Dependent Changes in FasL (CD95L) Modulate Macrophage Function in a Model of Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 2013;54:5321–31.
24. Zacks DN, Boehlke C, Richards AL, Zheng QD. Role of the Fas-signaling pathway in photoreceptor neuroprotection. Arch Ophthalmol 2007;125:1389-1395.
25. Besirli CG, Chinskey ND, Zheng QD, Zacks DN. Inhibition of retinal detachment-induced apoptosis in photoreceptors by a small peptide inhibitor of the fas receptor. Invest Ophthalmol Vis Sci 2010;51:2177-2184.
26. Huckfeldt RM, Vavvas DG. Neuroprotection for retinal detachment. Int Ophthalmol Clin 2013;53(4):105-17.