Causes
Many of the organic and iatrogenic etiologies need to be ruled out as the cause, and the following are several considerations: ocular surface disease; anterior basement membrane dystrophy; other irregular astigmatism; surgically induced astigmatism; posterior astigmatism; extremes of axial length/IOL prediction errors; and IOL tilt.
If a patient presents with residual astigmatism after the implantation of any IOL, surgeons should first examine his preoperative and postoperative keratometry values to determine whether the astigmatism is naturally occurring or surgically induced.
If the amount of astigmatism present after surgery is different from the preoperative amount, it may be surgically induced astigmatism. In these cases, we would look at the preoperative and postoperative keratometry readings and evaluate for any astigmatism induced by the incision. This can be determined just by looking at the keratometry readings.
Another likely cause of residual astigmatism is posterior astigmatism, which has been shown to significantly influence corneal astigmatism in certain cases.1 Even if posterior astigmatism was not identified or measured preoperatively, surgeons may be able to find evidence of it in after-the-fact analysis of a patient’s corneal imaging. A groundbreaking study found that ignoring posterior corneal astigmatism may yield incorrect estimation of total corneal astigmatism.1 The study included 715 corneas of 435 consecutive patients, and the mean amount of posterior corneal astigmatism was -0.30 D. The steep corneal meridian was aligned vertically in 86.6 percent of eyes for the posterior surface and in 51.9 percent of eyes for the anterior surface. As patients aged, the steep anterior corneal meridian tended to change from vertical to horizontal, while the steep posterior corneal meridian stayed the same. When the steeper anterior meridian was aligned vertically, the magnitudes of anterior and posterior corneal astigmatism were correlated; however, this was not the case when the steeper anterior meridian was aligned horizontally. According to the study results, “ … anterior corneal measurements underestimated total corneal astigmatism by 0.22 @ 180 and exceeded 0.5 D in 5 percent of eyes.”
 |  |
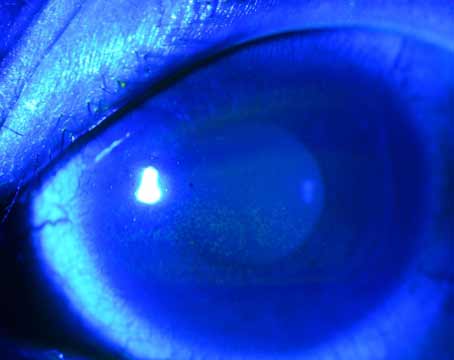
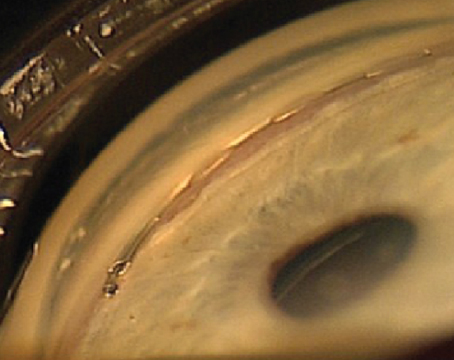
| Figures 1 and 2. Topographies showing irregularity and 2 D of astigmatism from anterior basement membrane dystrophy (left) and reduction after superficial keratectomy (right). | |
IOL Position
If the residual astigmatism cannot be attributed to the conditions mentioned above, it is time to examine the IOL to see if its position is causing residual refractive error.
If the patient has been implanted with a standard monofocal IOL and she has residual astigmatism, then the surgeon needs to determine whether the patient has residual astigmatism that was expected because of pre-existing corneal astigmatism or now has astigmatism not identified by keratometry preoperatively before the monofocal IOL implantation. If none of the previously mentioned causes of astigmatism can be identified, surgeons should examine the placement of the IOL, because tilt with a standard monofocal IOL can induce astigmatism. Several patients have been referred to me for evaluation of postoperative astigmatism only to find that they had one haptic in the bag and one haptic in the sulcus. This was not caught when the implant was placed, and it tilts the IOL, causing postoperative astigmatism in their refraction.
Accurate alignment of toric IOLs inside the eye remains challenging for numerous reasons, and misalignment has significant consequences. For every degree that the lens is off, the patient loses 3.3 percent of astigmatism correction. In other words, if a toric lens is off by 30 degrees, it has no cylindrical effect. Toric IOLs have their own nuances in terms of evaluating for residual astigmatism because they are designed to correct a patient’s naturally occurring astigmatism. Measuring the exact axis of astigmatism, as well as aligning a toric IOL to that axis can be somewhat imprecise. If you factor in the unpredictability of surgically induced astigmatism and effective lens position, residual astigmatism is not uncommon after toric IOL placement.
Treatment
If ocular surface disease has been found to be the culprit of residual astigmatism, it can be treated medically. Artificial tears, lid treatments, cyclosporine, punctal occlusion and even steroid drops can be used to control ocular surface inflammation. This is the most common cause of residual astigmatism in patients with standard monofocal IOLs, and treating with lubrication and tears can really help considerably to get back to that preoperative baseline.
A recent study found that cyclosporine 0.05% is an effective treatment for dry eye after cataract surgery.2 The study included 32 newly diagnosed patients with dry-eye syndrome. One week after cataract surgery, these patients received a twice-daily treatment of cyclosporine 0.05% for one eye and normal saline 0.9% for the other. Over time, both groups experienced an increase in Schirmer test 1 and tear-film breakup time. The eyes treated with cyclosporine demonstrated a significant increase in Schirmer test 1 at two months and an increase in TBUT time at two and three months. Additionally, the dry-eye symptom score was significantly reduced in the cyclosporine group.
In cases of anterior basement membrane dystrophy, superficial keratectomy can be very effective in treating the astigmatism. Additionally, a study has found that simple mechanical debridement achieves results that are comparable to those of other procedures used to treat anterior basement membrane dystrophy.3 This study included 74 eyes of 55 patients who were treated with mechanical epithelial debridement during a 15-year period. Patients’ mean age was 74 years, and most (80 percent) were women. Sixty-one eyes experienced visual difficulty before the procedure, and erosion symptoms alone were noted before the procedure in the other 13 eyes. At an early follow-up visit, mean best-corrected visual acuity had improved from 20/44 before surgery to 20/30. At the last follow-up visit (mean: 33 months), mean BCVA was 20/33. The mean refractive spherical equivalent changed -0.6 D (range: -4.75 D to +2 D).
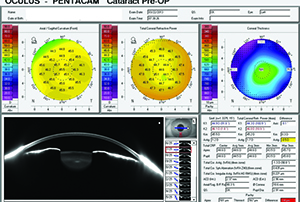 |
| Figure 3. Pentacam showing 0.5 D of additional cylinder contributed by the posterior cornea. |
For a standard IOL that is tilted, surgical repair with repositioning of the IOL is required. A recent study found that a tilted scleral-fixated IOL behaves like a toric IOL.5 Therefore, the astigmatism caused by the position of the scleral-fixated IOL could increase or decrease total astigmatism. This study included 26 eyes of 26 patients. The average amount of tilt was 2.25° ±1.93°, and the average amount of decentration was 359.28 ±194.70 µm. There was a positive and moderate relationship between tilt and astigmatism caused by the position of the IOL; however, there was no relationship observed between decentration and astigmatism caused by the position of the IOL.
For toric IOLs, first and foremost, any medical conditions will need to be treated. These lenses are more likely than standard IOLs to have alignment issues. If a patient has an extreme axial length, a very short eye or a very long eye, the prediction values for toric implants can fall off due to prediction errors of effective lens position and the resulting correction of astigmatism at the lenticular plane. Some of the toric calculators account for axial length, and some do not. Even those that do often use a standard or generalized conversion factor for the degree of astigmatism correction at the corneal plane. If a patient has 1 D of astigmatism at the cornea, it cannot be corrected with 1 D of astigmatism at the lenticular plane. There is a conversion factor that comes into play based on effective lens position that is standardized across most IOLs, regardless of power of the lens. However, if the patient has a very long or a very short eye, the power of toricity may not be sufficient or may be too much and may under- or overcorrect. With toric lenses, it is especially important to consider the posterior cornea. If it wasn’t examined preoperatively, the posterior cornea can add or subtract up to 1 D of astigmatism, which can also be the source of residual cylinder.
If a patient with a toric IOL has residual astigmatism, it can generally be corrected by rotating the IOL, laser vision correction, IOL exchange or LRIs. If a toric IOL is not correctly aligned, it will need to be repositioned. We use topography, tomography and keratometry to determine the axis of alignment. Unfortunately, none of these measurements are ever identical, so we always have competing preoperative values, and we often are questioning the exact axis. A recent report found that even toric IOLs that are
positioned appropriately intraoperatively can benefit from rotation.6 The report included three eyes of two patients. Using an online toric IOL calculator, it was determined that IOL rotation would significantly improve astigmatic outcomes. Two months after IOL rotation, residual manifest astigmatism was 0.5 D, 0 D, and 0.75 D in the three eyes, showing that the use of the toric IOL online calculator maximized the uncorrected visual and refractive outcomes.
These online programs (e.g., astigmatismfix.com) that have been developed look at a patient’s refraction and the current power and axis of the toric lens and plug that into vector analyses. They can tell surgeons whether there is a new ideal axis of alignment. Perhaps all of your keratometry values were off by 5° or 10°, and you weren’t sure which one was the right number. Now, looking at those vector analyses postoperatively, we can determine which one was probably the right number. Plugging these numbers into a calculator, such as the one on the ASCRS website, will help give you a better sense of the ideal axis of the lens and to what degree you are going to reduce the residual astigmatism. REVIEW
Dr. Kieval is the director of cornea, cataract and refractive surgery at Lexington Eye Associates. Contact him at jkieval@gmail.com. He is a consultant for Alcon, Allergan, AMO and Shire.
1. Koch DD, Ali SF, Weikert MP, Shirayama M, et al. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg 2012;38:2080-2087.
2. Chung YW, Oh TH, Chung SK. The effect of topical cyclosporine 0.05% on dry eye after cataract surgery. Korean J Ophthalmol 2013;27:167-171.
3. Itty S, Hamilton SS, Baratz KH, Diehl NN, Maguire LJ. Outcomes of epithelial debridement for anterior basement membrane dystrophy. Am J Ophthalmol 2007;144:217-221.
4. Lim R, Borasio E, Ilari L. Long-term stability of keratometric astigmatism after limbal relaxing incisions. J Cataract Refract Surg 2014;40:1676-1681.
5. Polat N, Devranoglu KK, Ozdamar MA, Arici C. Evaluation of scleral-fixated intraocular lens position anomalies by anterior segment optical coherence tomography. Turk J Med Sci 2014;44:1118-1123.
6. Lockwood JC, Randleman JB. Toric intraocular lens rotation to optimize refractive outcome despite appropriate intraoperative positioning. J Cataract Refract Surg 2015;41:878-883.