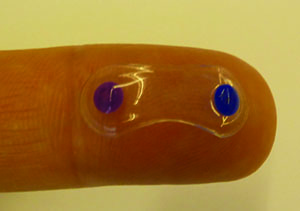
Amorphex Therapeutics
Amorphex (Andover, Mass.) has combined polymers that allow sustained release of drug with a device platform called the Topical Ophthalmic Drug Delivery Device to create a sustained-release modality that floats on the tear film beneath the lid. The device without the drug has been tested for tolerability in humans, and the company is currently securing funding to perform a Phase I trial.
Robert Thompson, president and chief executive officer of Amorphex says the TODDD material matrix can be modified to achieve the proper balance of drug loading and release. Through the process, the selected drug is polymerized directly into the material. So far, most of the company’s work has focused on timolol and prostaglandins.
In a formal clinical study of the device’s tolerability, researchers placed it beneath the lids of 20 subjects at the New England College of Optometry. “We had the subjects wear it on a daily-wear basis, and then transition to a 30-day, uninterrupted wearing of it,” explains Mr. Thompson.
|
If a subject doesn’t experience movement of the device initially, chances are it will be able to sit there unnoticed, says Mr. Thompson. “The only time you’ll feel it, not feel pain, but literally just be aware of it, is when you do an upward look,” he says.
The company has also placed timolol-laden TODDDs into the eye of a glaucoma patient over a six-month period and monitored the intraocular pressure response. “The timolol devices averaged an IOP reduction percentage of 16 to 22 percent,” says Mr. Thompson. “This is consistent with the reduction you’d expect from timolol drops. However, the subject was being exposed to 15 to 20 percent of the drug he’d get from drops since, with drops, a good amount goes down the tear ducts and nasal passages and is swallowed.”
One of the potential benefits of the device is that it doesn’t necessarily need to be placed or removed in an eye-care provider’s office. “If the patient is at an assisted-care facility, you could teach a nurse or another employee how to insert and remove it in 10 minutes,” says Mr. Thompson. “In our clinical trial, we taught all the subjects how to remove it.”
As mentioned, the company is in the process of securing funding for the device’s next step, a Phase I trial. “The Phase I trial will be with drug,” says Mr. Thompson. “In it, we’d probably be limited to 90 days maximum but, in the case of glaucoma, that’s certainly long enough to monitor the IOP.”
Ocular Therapeutix
Ocular Therapeutix (Bedford, Mass.) has a sustained-release dexamethasone punctal plug delivery system that’s in Phase III trials for post-cataract pain and inflammation; it may also be a viable way to deliver glaucoma medications over an extended period. The company is currently enrolling patients for a Phase IIb trial of a punctal plug designed to release travoprost for a period of up to 90 days.
In addition to tackling compliance problems and issues with patients limiting their doses to save money on drug co-pays, Amar Sawhney, PhD, president, chief executive officer and chairman of Ocular Therapeutix, says a sustained-release system can also help overcome side-effect problems. “These drugs often have side-effect profiles,” Dr. Sawhney says. “One aspect is they can have preservatives that can interfere with the long-term health of the ocular surface. Second, one peak-dose effect of prostaglandin analogs is a hyperemia that occurs in 20 to 40 percent of patients, depending on the agent that’s used.
“Approaches were tried in the past, such as Ocusert,” Dr. Sawhney continues. “This was placed in the upper fornix and released pilocarpine. However, it was a fairly decent-sized disk and the problem was it didn’t stay in place very consistently, and fell out or was uncomfortable. So, while it worked, it didn’t catch on enough due to these drawbacks. We felt there had to be an approach that lets you deliver this medication over a meaningful duration—for us that was two to three months—in a non-invasive way. We didn’t want to put needle holes in people or have to perform implant surgery in anyone, because that clearly wouldn’t be a great option if you’re going to be doing it every two to three months.”
|
Ocular Therapeutix conducted a Phase II study outside the United States that showed an intraocular pressure decrease similar to travoprost drops, but Dr. Sawhney says it wasn’t statistically powered. The company is currently enrolling a Phase IIb study in the United States that will look at the size of the effect and pave the way for a Phase III trial. The Phase II trial did help them learn more about the device, however. “There are some adverse events, but nothing that’s unusual for glaucoma therapy, and nothing that appears to be serious,” says Dr. Sawhney. “What you do get is possibly some localized itching for a short duration because a prostaglandin is a pro-inflammatory drug. But, you don’t get hyperemia, which can occur with prostaglandins and Rho kinase inhibitors up to 40 percent of the time. You don’t get the hyperemia because you’re releasing the drug in a steady fashion.”
As with anything inserted through the punctum there’s a risk of ejection. “We have done a number of non-significant risk studies,” says Dr. Sawhney. “In these, we take placebo plugs and optimize their design, looking at shape, profile, means of insertion, hardness/softness and different physical dimensions in order to optimize retention. In these studies to date, we’ve seen up to 92-percent retention.”
Another thing physicians, and regulators, are on the lookout for with punctal plug delivery systems is the possibility of systemic dosing of the drug. “We actually had to do a clinical study looking at that for the dexamethasone plug,” says Dr. Sawhney. “I can definitely say that we didn’t see [systemic levels] with that device. In the prostaglandin work, we haven’t done that yet, but we don’t expect to see systemic levels because the amounts are generally quite tiny and released over a continuous period of time.”
Ohr Pharmaceutical
Ohr Pharmaceutical’s (New York City) plan to overcome compliance issues with glaucoma medication is to inject micro- or nanoparticles into the eye that would then release a glaucoma drug/drugs over an extended period of time. However, the existing emulsion methods make it difficult to use narrow-gauge needles to do this. In response, Ohr has developed a new template fabrication method for these particles that makes it easier to get them into the eye. The company is developing delivery approaches for primary open-angle glaucoma as well as steroid-induced glaucoma.
“A big advantage of our template approach is its control of particle size, resulting in greater uniformity,” explains Nikita Malavia, PhD, senior scientist at Ohr Pharmaceutical. “Currently used emulsion-based methods generate a very broad particle size distribution, meaning that small micro- or nanoparticles are mixed in with the large ones, making it difficult to deliver them through small-gauge needles, as well as creating greater variability in drug release. In addition, emulsion-based methods are limited as to the type of polymer that you can use in the process, as well as the amount of drug that can be loaded into these formulations. Since sustained drug delivery is contingent upon the properties of both the biodegradable polymers as well as the amount of loaded drug, a proper balance between the these two factors is required for efficacious dosing for an extended period of time. Emulsion-based methods usually don’t allow more than 10 percent by weight of drug loading. If you wanted to replace monthly injections with an injection every three to six months, you typically need to have a greater than 10 percent drug load. Our technology allows the incorporation of much more than 10 percent of drug by weight, and we’ve been able to incorporate more than 50 percent by weight of some molecules. This means that as much as half of the actual formulation going into the eye is drug, and just the remaining half is polymer, which makes for much more efficient ocular drug delivery.”
Dr. Malavia describes the process: “Our process uses microfabrication technology, which is similar to what is currently used in the semiconductor industry,” she explains. “We use a silicon wafer master template, that has our customized pattern on it, onto which we pour a hydrogel material that we then let cure. We then fill this hydrogel template with our formulation, which includes both polymer and drug. We use a solvent to dissolve away this hydrogel, leaving just the uniformly sized and shaped microparticles as a result. The pattern we lay down on our wafer controls the size of our final micro- or nanoparticles. So, if we want a nanoparticle size, we’d pattern down nano-range features on our wafer. If we want microparticle size, we’d pattern down micron-sized features.”
Dr. Malavia says the fabrication process allows a unique layering approach different from other microparticle processes. “We can control the initial release of drug from the formulation by using an approach called layering. We can put down a layer of one sort of polymer that degrades slowly or quickly, and then follow with another layer with the drug, and finally layer with a polymer without drug. In this way, we create a sandwiched microparticle formulation of polymer and drug layers. For some indications, we might want a loading dose which requires high initial release, while in others we might need a lower initial release. We can provide both types of therapies with the layering process.” She says it’s possible to load more than one drug into the same particle using the layering process as well.
Ohr is currently testing both injectable and topical glaucoma drugs alone, without them being put into a polymer, on humans, and is testing the drug/polymer injection on animals. The company is also experimenting with different injection sites. “We’ve already taken two compounds forward in glaucoma, one hydrophilic and one hydrophobic,” says Dr. Malavia. “We’ve been able to achieve release for four to six months with these therapeutic agents. We’re not limited as to the type of polymer nor the targeted particle sizes in our formulation process, so we can create designs around a whole list of parameters to achieve the desired effective release profiles. As a result, we are performing preclinical animal studies and hope to start human clinical trials next year.”
Another company pursuing the injectable route is Envisia Therapeutics (Research Triangle Park, N.C.), which uses biodegradable particles to deliver travoprost. In a canine study, the microparticle injection reduced IOP by an average of 35 percent (6.4 ±0.6 mmHg) over eight months, with the pressure reverting to baseline at nine months. (Navratil T, et al. IOVS 2015;55:ARVO E-Abstract 5706) The company is currently planning a Phase II study.
pSivida
As part of a collaboration agreement with Pfizer, pSivida (Watertown, Mass.) is investigating the use of its Durasert implantable, bioerodable technology to release latanoprost over an extended period of time, possibly six months to a year. Durasert is the same technology licensed for use by Alimera Sciences’ Iluvien implant for diabetic macular edema. The Iluvien implant is 3 mm long, 0.5 mm wide, and is injected through a 27-ga. needle. Pfizer and pSivida are currently conducting a Phase I/II safety and efficacy trial of the latanoprost implant in patients with elevated intraocular pressure.
|
In terms of the time period, and the possible objections patients might have to getting an injection rather than just putting in drops, Dr. Ashton offers a unique perspective. “Being a glaucoma patient myself, I’ve got something of an inside track on that,” he says. “Like most patients, I see my physician every six months, so a treatment that doesn’t last at least six months isn’t going to be very interesting. I’d be fine having an injection in my eye once a year. It would make my life easier because I tend to leave my Xalatan bottles scattered throughout various hotels since I travel a lot.
“So, a six or 12-month implant would be the goal,” Dr. Ashton continues. “It should also be bioerodable, but that’s not an issue. However, the key to the erosion isn’t to control the release rate of the drug, but to dispose of the used device so the eye doesn’t become a graveyard of used-up devices. The project is kind of unusual because we already have a pretty good idea of the drugs that are required and the release rate that’s required to be efficacious, which are normally guesses in most of the other projects that we’ve been working on.”
Dr. Ashton says he can’t provide results from the ongoing Durasert/latanoprost study, but has observed that the implant appears to be stable, and there have been no instances of conjunctival erosion over the implant. Since combinations of drugs for glaucoma are also a popular option for certain patients, he says future trials may look at combining agents. “Combining them is definitely possible,” he says. “It gets tricky with respect to clinical trial design. The FDA would expect to see evidence that the combination is more effective than either drug alone.”
Down the line a bit, Dr. Ashton thinks one of the more exciting applications of the technology would be not just combining two pressure-lowering drugs, but combining a pressure-lowering drug with a neuroprotectant. “That’s the sort of unknown frontier for glaucoma,” he says. “Because glaucoma, as we know, isn’t really a pressure disease per se. Rather, it’s an atrophy, or death, of the optic nerve. So how do you treat that? Now we have a technology that, from a single injection, can provide drug delivery for up to three years, so there’s the potential to actually have a neuroprotectant in there as well.” REVIEW