|
Thanks in part to the economic downturn that began in 2008, research funding in the United States has dropped dramatically in recent years, taking a toll on the amount of research being done and putting intense pressure on researchers. Here, five individuals with firsthand experience in the field share their thoughts on this situation, their advice for those hoping to go into research (or just wanting to support it) and their hopes for the future.
Running Out of Gas?
“We’re living in a golden era for biomedical research, particularly in ophthalmology,” says Russell N. Van Gelder, MD, PhD, chairman of the department of ophthalmology at the University of Washington and current president of the American Academy of Ophthalmology. (Dr. Van Gelder is an active clinician-scientist; his National Institutes of Health-funded laboratory has been at the forefront of research into non-visual ocular photoreception and ocular inflammatory disease.) “The sizeable investments the government has made in basic research over the past 50 years—in particular the doubling of the NIH budget in the 1990s and early 2000s—have put us in a position where our basic science is ripe to be translated to direct patient care. The frustrating thing is that, just as we’re ready to harvest the crops, there’s no gas in the tractor.
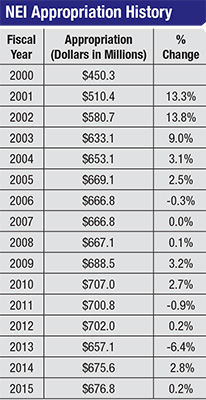
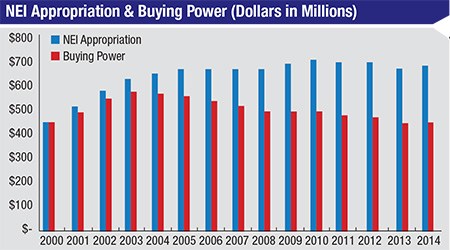
“Mostly flat funding of the NIH over the past decade—with the exception of a one-time bolus of money that was part of the stimulus package in 2009—combined with the impact of biomedical research inflation, has equated to a huge cut in effective funding,” he continues. [See chart, p. 24] “The size of modular grants has been capped at $250,000 by the NIH, and that hasn’t changed in a long time. But $250,000 today does not buy any-where near as much research as it did 10 years ago. Additionally, the NIH has set aside a good portion of its budget for very specific projects, such as the Brain Mapping project, which has reduced the available set of funds for traditional investigator-sponsored RO1-type research [the original and historically oldest type of NIH grant offered for health-related research]. Meanwhile, changes in industry, such as increased consolidation among the largest pharmaceutical companies, have decreased the amount of support that the independent investigator is seeing from industry. The final piece of the perfect storm is that the regulatory environment has gotten more stringent; the amount of time and overhead that goes into regulatory work in order to do research has become more and more onerous over the past decade.”
“Academic departments and academic medical centers are feeling tremendous pressure on all fronts,” agrees Christopher A. Girkin, MD, MSPH, chairman of the Department of Ophthalmology at the University of Alabama at Birmingham and chief medical officer of the Callahan Eye Foundation Hospital. “We’re seeing a simultaneous erosion of many of the traditional sources of income that support an academic enterprise. We have reductions in the federal government’s budget for research—by far the largest source of support—along with a reduction in clinical revenues, which has reduced the ability to fund the educational and research components of the academic mission. In addition, concerns over market stability may lead to reduction in support from charitable foundations as they seek to preserve their endowments.
“The result is that you see talented people and great projects not getting funded,” he says. “This is tragic to the researchers and frustrates our efforts to find new cures. This frustration is felt at all levels, by the program officers at the NEI, by leaders in academia, and most importantly by the research faculty and those critically important young minds that may choose alternative career paths.”
Dr. Van Gelder notes that part of the problem, at least from the perspective of industry, is the barriers to approval erected by the Food and Drug Administration. “The FDA’s bar for approval is so high for many drugs, and the cost of going into clinical trials is so incredibly high, that the thought of developing a drug for a small market or a niche is no longer economically feasible for a large drug company,” he says. “If the drug isn’t going to sell $500 million to a billion dollars a year, they can’t afford to go down that path. This really decreases the amount of discovery that’s happening associated with many diseases that might not be billion-dollar-a-year kind of markets.”
“Those three factors together—the decrease in federal support, the decrease in industry support and the increased overhead when doing research (especially regulatory and administrative overhead)—are making it much more difficult to achieve the kind of progress that resulted in great advances like the game-changing anti-VEGF drugs. Those drugs started as NIH-funded research many years ago, and they have obviously transformed our clinical world. Given current conditions, I think we’ll be waiting longer for the next transformative discovery to emerge from basic research.”
Choices and Consequences
“The economic system in our country is going through a very tough time, and this is partly responsible for our failure to invest adequately in research,” says Paul Sieving, MD, PhD, director of the National Eye Institute. (Dr. Sieving is a lifelong clinician-scientist and former professor of ophthalmic genetics at the University of Michigan Medical School.) “We are depriving many kids of an adequate education while allowing others to graduate from medical school $150,000 in debt. The downturn in research funding is just part of a ship that’s taking on water. On the other hand, one must feel optimistic for the Chinese, because they are investing in research heavily. We may be asking ourselves a few years from now how the Chinese got ahead of us. The answer will be our loss of willingness to fund basic research at the full level.
|
“I don’t want to paint the situation as bleak, but I do think we have to be honest about the state of affairs,” he says. “To put things in perspective, this country spends twice as much on soft drinks every year as we spend on the NIH budget. This has to change. How badly do we want cancer therapies or therapies for heart disease? How badly do we want to develop ocular gene therapy to treat blinding eye disease?
“Meanwhile, we have an explosion of opportunity,” he says. “A series of breakthroughs starting around the year 2000 was seminal for our fundamental understanding of human disease, courtesy of elucidating the human genome. Before that, many genes had been identified one at a time, but with the completion of the Human Genome Project we had access to all the genes in the human body, and diseases became understood as having a genetic basis or predisposition. In 2005 vision researchers uncovered gene variants for complement factor H that substantially increase the risk of developing age-related macular degeneration. (Of course, the story is complex, as currently nearly 25 genes are known to interact and contribute to the risk for developing AMD.) Then another fundamental breakthrough came in 2008 with the first successful human gene therapy for treating one form of Leber’s congenital amaurosis—childhood blindness, which had been found to result from mutations in the RPE65 gene. Clearly, genetics is providing remarkable tools to understand disease, and soon, to treat disease. Research is making that possible.
“I’ve been researching one particular disease, X-linked retinoschisis, for 30 years,” he adds. “Just last month I oversaw the first injection of gene therapy for this disease—one of the first gene therapy trials of this type ever mounted at NIH. We’re very excited to see what the results will be, but the reality is that it took 30 years to reach this point. Added up, that’s a lot of time and work and money. The same is true of the superb work being done by people such as Russell Van Gelder. His clinical team at the University of Washington is collaborating with a strong basic research team at Berkeley, working on light-gated channels to restore vision to a blind eye. That research funding began 10 years ago, and it will take additional work before it is tested in a human being. That’s an enormous amount of effort and money.
“The reality is, there are no shortcuts,” he says. “Meanwhile, there are some 180 genes known to cause photoreceptors to die. Each of those may require a different treatment approach. Well, I don’t see 180 laboratories working on that yet. We have a long way to go.”
Survival Strategies
With decreasing availability of grant money, researchers are trying to find new ways to make their grant proposals more likely to be approved. “Times have gotten tough as the economy has changed,” says clinician-scientist Natalie A. Afshari, MD, a professor of ophthalmology and chief of cornea and refractive surgery at the Shiley Eye Institute, University of California, San Diego. (She currently has an NIH grant to study Fuchs’ corneal dystrophy.) “Grants were funded at a greater rate in the past. Today, most scientists in our field believe that you either have to improve your chances of getting funded by submitting more grants, or you have to have a truly great grant proposal with a lot of nice data to back it up. If one out of 10 grants is being approved, then submitting 10 proposals should give you a reasonable chance of having one approved—assuming all of them are excellent proposals. But even in the best scenario, there’s always a chance that you won’t get funding. In fact, the average age at which people get their first grant from NIH has gone up over the years; today, you have to have more experience and better evidence to get funded.
“Luckily, there are some great people at the NEI who have worked hard to keep funding for research available,” she continues. “We’re very grateful for their support. The director, Paul Sieving, MD, has been a great advocate for scientists; as a clinician-scientist himself, he knows what everyone goes through. The Institute puts some focus on high-priority diseases, such as certain aging-related diseases that would particularly benefit from progress in our understanding. This increases the likelihood of proposals in those areas being funded. The Institute even invites proposals in those areas.”
Dr. Girkin suggests another strategy. “To continue to be funded in this environment, a researcher needs to have multiple diverse and overlapping sources of funds,” he says. “The approach we are taking is an adaptive strategy: cluster-hiring of researchers who have interdigitated skill sets that enable multi-investigator research programs. I’m aware that some researchers are trying to maintain their funding by submitting a much greater number of proposals. While I’ve seen this shotgun approach done successfully in a few situations, I don’t think that’s a good long-term strategy, and it’s not a sound way to steward our societal investment in research. If you can pick a few key areas in which you can lead, and invest in those areas to keep those labs moving forward, I think you will continue to thrive as a department.
“I’ve had the opportunity to work with a number of people, both in basic research and clinical research, in interdepartmental collaborations across schools and boundaries,” he continues. “To me, working with a team is more satisfying. The fun of science is when you can engage lots of different people who approach the same problem with completely different skill sets. Teams like that can create studies that are long-lasting and secure, and I believe that building and protecting this kind of team is the best investment in these chaotic times. This is critical because there’s so much specialized knowledge that needs to be brought to the table to solve these complex problems.”
Not all suggestions about how to make the best of this reduction in grant funding, however, are equally helpful. “I arrived at NEI in 2001,” recalls Dr. Sieving. “Early on, I was introduced to a new word in our NIH meetings: ‘efficiency.’ We heard that research must be more efficient. Unfortunately, research, by its nature, is not efficient. Research takes lots of effort and lots of time to think creatively, plus good old-fashioned elbow grease. It involves trying novel strategies and running into many dead ends, and then doing more work on all of the nuts and bolts that make things come together. Efficiency, by itself, is not the answer.”
What About the Next Generation?
As the difficulty of getting grants increases, it appears to be dampening the enthusiasm of potential research candidates. “I do think we’re seeing fewer people choosing to go into research at the graduate level,” says Dr. Girkin. “You see students shifting out of PhD programs, going into industry or some other track. I think as we see the economic realities hitting home, academia is going to lose some of this talent if we don’t move to increase support for research.”
Dr. Sieving notes that he doesn’t have to explain the situation to young people entering the field. “The young people see the problem for themselves,” he says. “They are working in labs and see their mentors, or the lab next door, running into funding problems. They get the message.”
Dr. Afshari says there’s no problem getting good people to work on research projects. “However, fewer people are going into research as principal investigators,” she says. “That’s true for several reasons. For one thing, it’s much easier to simply see patients. When you work with a patient, the results are right in front of you. When you write a grant and put your whole heart and soul into it, the chances of it getting funded are still not that high. There’s also the financial reality that clinicians make more money than clinician-scientists. As a result, there are fewer people initiating research projects.”
Despite these circumstances, Dr. Van Gelder says he still sees great enthusiasm among young physician-scientists and researchers about going into research. “This is still a way to have an enormous impact,” he notes. “Using your insights to improve the world is a very attractive career ambition. Being able to think for a while, do a few experiments and make the world a better place—that’s still a great aspiration.
|
“I feel bad for many of our graduate students because I know that they won’t have jobs in academia waiting for them when they finish,” he adds. “There are jobs in industry, but those jobs are becoming fewer as more companies that do not value Research & Development enter the marketplace. A couple of those companies are now in ophthalmology, and they have succeeded in dismantling what were good research departments within major companies. So there is a trend in the field toward decreasing investment in research, which is very unfortunate.”
In the meantime, Dr. Van Gelder says he still encourages people to go into research. “When I was a graduate student during that last contraction 25 years ago, Arthur Kornberg, MD, who was a Nobel Prize winner and the chairman of our biochemistry department, sat down with the grad students to give us a little pep talk. We thought he was going to tell us that things would get better—but he didn’t. He said, ‘Things are bad now; but compared to when I started in the 1940s, things are so good that they’re unrecognizable to me.’ He pointed out that things we could easily do in the 1990s would have taken months or years when he was starting out. He told us to keep a long-term perspective.
“I still agree with that,” Dr. Van Gelder concludes. “No one is going to get wealthy doing basic research; there are other ways to make a living that are more lucrative. The effort that goes into basic research is enormous, and the stakes are high because you’re testing hypotheses that may turn out to be incorrect. But ultimately it’s a deeply satisfying career and a wonderful way to make essential contributions to the world.”
Dr. Girkin feels similarly. “Despite all the challenges, I still encourage clinicians to go into research,” he says. “We have much to contribute, and research makes you a better clinician. It also brings many intangible benefits, resulting in a more fulfilling career. Lastly, if clinical reimbursements continue to go down, it may turn out to be financially wise as well—a way to diversify income sources.
“However, in the end, research has to be something you can get passionate about,” he says. “You need to find lifelong questions to try to answer. That’s the key. I believe that if you can develop that, you can be successful, whatever environment we create for ourselves in the future.”
Dr. Sieving also continues to strongly encourage young people to go into medical research. “I’m positive about the science side, and I’m positive about the opportunity to break new ground medically,” he explains. “It’s truly miraculous where we’re headed. But we have a long road ahead to get from here to there in curing and even preventing disease.”
Fighting for Federal Funding
James F. Jorkasky is executive director of the National Alliance for Eye and Vision Research, which works with the vision community to advocate with Congress to ensure that vision research funding remains a priority. “In our traditional role as the ‘Friends of the NEI’ and advocates for vision research funding, we work hard to get as much congressional funding as we can,” he says. “However, it’s a very tough environment right now because of the budget caps and the sequester. That’s certainly true in terms of the NEI budget. As you probably know, NEI took a $36 million sequester cut in fiscal year 2013, and it’s still down about $25 million from the pre-sequester fiscal year 2012 level.”
Mr. Jorkasky says that members of Congress on both sides of the isle appreciate the value of vision research. “I think there’s generally a great deal of support for biomedical research, no matter what party you’re in,” he says. “Nevertheless, it’s going to continue to be a tough funding environment, unless Congress comes up with some kind of a new bipartisan formula to arrive at the deficit-reduction goals they wish for—a formula that would allow for increases in certain programs that they’d like to be better funded. In the meantime, we’re on the Hill and we’re asking for an increase, and we’re asking that the NIH and NEI be waived from the Budget Control Act caps. The entire health-care community, whether you’re an advocate for vision or lupus or Parkinson’s, is asking for relief from sequester and budget control act caps.
“One of the things we’ve done at NAEVR in the past few years is to get federal research funding for vision anywhere we can from other federal agencies,” he continues. “I always think of it in terms of: What are we getting for the vision researchers out there? NAEVR advocacy resulted in Congress creating the dedicated Peer Reviewed Vision Research Program within the Department of Defense appropriations in fiscal year 2009. Since that line item was created, the DOD has awarded 60 grants to vision researchers, totaling $45 million.”
Mr. Jorkasky points out that this impacts the overall funding picture. “Take fiscal year 2013, for example,” he says. “The NEI’s budget was cut by $36 million, but in that funding cycle, the DOD awarded $15 million to vision researchers—albeit for very specific, penetrating eye injury and traumatic brain injury-related visual dysfunction research. That $15 million made up almost half of what the NEI lost. Although this isn’t the type of research that NEI would typically fund—because the DOD research is very specific to traumatic eye injuries on the battlefield—the discoveries that are made relating to retinal and cornea protection or visual processing in traumatic brain injuries may ultimately have potential civilian applications. For example, they may help people who are in car accidents, or suffer concussive injuries to the head such as we are seeing in professional sports.
“Another source of funding for vision researchers that has appeared in the past year is the NIH’s Brain Initiative,” he continues. “In fiscal year 2014, the program awarded $46 million in funding. Eighteen of 58 grants went to NEI-supported primary investigators, and six other grants were vision-centered proposals, for a total of $22 million to vision researchers. The Brain Initiative budget for fiscal year 2015 is $65 million, and vision researchers can compete for that. Vision researchers have also gotten funding from other institutes at the NIH, such as the National Institute of Diabetes and Digestive and Kidney Disorders, the National Heart, Lung and Blood Institute and the National Institute on Aging.
“Let me be clear: My priority is always going to be getting as much NEI funding as we possibly can,” he says. “But in the meantime, let’s see what other sources of funding we can get out there. In fact, the NEI itself is quite good at working with the other institutes at the NIH to find opportunities for vision researchers to get funding through other routes. The NEI has always been very collaborative in that respect.
“In the interim, as a researcher, you have to be clever and look for other potential funding sources out there,” he adds. “You might have to look at the problem you’re trying to solve a little differently because of what that particular funding source may be asking of you, in terms of how does vision fit into brain studies, for example. But doing so may help you get the funding you need.”
Bright Spots
The outlook for vision research is definitely not all doom and gloom. “One bright spot is that there are a growing number of mid-cap and small-cap biotechs getting into eye research,” notes Dr. Van Gelder. “That’s a very encouraging sign, although I don’t think it’s a substitute for the kind of resources the big companies bring to the table. Another positive change is that some research can be done for less money overseas, which may reduce costs to some extent. That has allowed some smaller companies to engage in Phase III clinical trials, which they couldn’t afford to do in the United States.
“Another positive sign is that big data is now becoming available—in particular, mining of registry data as an alternative to doing large clinical trials,” he continues. “It’s very clear that if you capture all of the clinical data out there, you don’t really have to worry about conducting studies to answer many postmarketing or clinical trial questions; you can answer them out of that data set. That is a bright spot on the horizon, and if I were advising young trainees now, I would advise them to look carefully at tooling themselves for working with big data—being able to analyze and mine large datasets for research.”
Dr. Afshari notes that improvements in technology have changed the nature and speed of research. “For example, I do genetics research,” she says. “The cost of genotyping has really come down in recent years; we can now look at thousands of different genes for what it cost to look at a few hundred a few years ago. Computerization has also made things faster and easier. Mass production technology and robotics have eliminated some laboratory procedures that had to be done painstakingly by hand in the past. And our measurements are far more accurate than they were even a few years ago. So technology has made it easier to do the research.
“Those improvements in technology have created more research possibilities in our understanding of the underpinnings of a disease,” she continues. “People have bigger ideas. We think about doing things we couldn’t do before—like finding a gene that’s causing a certain eye disease. Before, where would you start? Now, we can look at a lot more genes, much faster and at a fraction of the cost. The fact that we can do larger research projects faster has enlarged our goals.”
“Everybody’s nervous about what the future holds,” adds Dr. Girkin. “But I think there’s a lot of opportunity as well. In building more efficient models of care, more research resources may still be brought to bear. Furthermore, advances in research methods have made today’s researchers and research teams increasingly more efficient. We’ll have to keep our eyes open so that we take advantage of these opportunities as they appear.”
Fighting the Good Fight
Clearly, vision research has the potential to benefit everyone. So: What can the average ophthalmologist do to ensure that this story has a happy ending? “It’s really important that we, as a group of people interested in supporting vision research, organize and lobby for a piece of what’s available,” says Dr. Girkin.
 |
“We’re currently advocating on the Hill for NIH/NEI funding increases and increased VRP funding at the Department of Defense,” notes Mr. Jorkasky. “It helps when the community gets onboard and advocates for those funding increases. Ophthalmologists can go through the Academy website and contact Congress; they can also go through NAEVR’s “Contact Congress” section of its website (eyeresearch.org)
as well. We have letters in there that they can send to their members of Congress indicating their support for an NEI funding increase. I think everything an individual physician does makes a difference. You have to keep the drumbeat up. Congress needs to continue to hear from us.”
In the meantime, Dr. Van Gelder sees plenty of reason for hope. “It’s clear that we’re not going to eliminate blindness just using the knowledge we have today,” he says. “We can’t reverse glaucoma; we really just slow down diabetic retinopathy and other diseases. The drugs we now have for macular degeneration are wonderful, but they don’t cure the disease and they don’t block the dry form.
“However, there’s hope that things will improve,” he says. “I believe the dedicated clinician-scientists and full-time scientists are still going to make the discoveries that change our landscape and largely eliminate blinding disease. There’s great opportunity out there. I like to tell our trainees that glaucoma and uveitis are both one good drug away from being cured.
“The pendulum swings back and forth,” he continues. “I’ve been doing this long enough to have gone through the last research funding contraction in the early 1990s, which was very painful; a lot of people left science during that time. But eventually, public policy makers understand that the only way to really improve things is to invest at the basic level, and then continue to invest enough to harvest the fruits of that research. We have to have faith that the political environment is going to change at some point in the future and encourage more investment in our health. As someone who lives in a democracy, you have to have hope that reason will ultimately prevail.”
Meanwhile, those who are currently doing vision research can help by continuing to encourage young ophthalmologists to consider becoming researchers and clinician-scientists. Dr. Afshari admits that being a clinician-scientist is a lot of work, but says the rewards are significant. “I do a lot of my research correspondence and writing between 4:00 and 6:00 a.m., even on clinic days,” she says. “It’s a lot of work, but there is nothing more satisfying. The life of a clinician-scientist is amazing, and I think most of us would say that we truly love what we do.
“It’s enjoyable to talk to collaborators and others about the challenges you’re trying to resolve,” she continues. “It’s wonderful to meet with a student trainee or postdoc and encourage their intellectual curiosity. Once people have done research, that analytical way of looking at a problem stays with them, even if they decide not to go into research. They learn how to think about data critically and it changes the way they approach patients with certain problems. And of course, we get to apply what we learn in the clinic. There’s nothing more satisfying than being able to have an impact, answering patients’ questions or changing the outcome of a disease, based on what one has learned.”
“I wish it were easier to find the capital and resources to do research,” adds Dr. Van Gelder. “However, the muse that drives people to do research is a noble muse and they should continue to follow it. I do; I still devote about half of my time to my bench research, and I wouldn’t give it up for the world.” REVIEW