Protocol T’s Background
John Wells, MD, of the Palmetto Retina Center in West Columbia, S.C., was first author of the study, and says the initial motivation for the research was to quantify Avastin’s efficacy in DME.
“Of course, ranibizumab was in use and bevacizumab was also available and cheaper,” Dr. Wells says. “But no study had shown that Avastin was effective in DME, except for one study from England that showed it was better than laser. So the DRCR Network saw the question of whether these drugs were equally effective in DME as an important one to answer. Aflibercept was approved for age-related macular degeneration treatment about eight months before the study started, and the network decided that once it got approved by the [Food and Drug Administration] and was available, that we’d include it in the three-way comparison. I’m glad we did.” The network and the study are funded by the National Eye Institute, the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institutes of Health. Regeneron provided the aflibercept, and Genentech the ranibizumab, for free. “This saved the network about $5 million,” says Dr. Wells. “If we had to pay for the drugs ourselves, it would have consumed every last dollar we had. However, the drug companies had nothing to do with the analysis; it was all the DRCR network.”
The study was conducted at 89 sites, over which 660 patients with diabetic macular edema were randomized to receive injections of 2-mg aflibercept (n: 224), 1.25-mg bevacizumab (n: 218) or 0.3-mg ranibizumab (n: 218). The drugs were administered as often as every four weeks. At the 24-week visit, regardless of visual acuity and macular thickness, the researchers would withhold the injection if there had been no improvement or worsening after two consecutive injections. The investigators would resume treatment if the visual acuity letter score or the retinal thickness worsened.
Study Results
It turned out the latecomer, aflibercept, made a difference for patients with certain levels of vision.
|
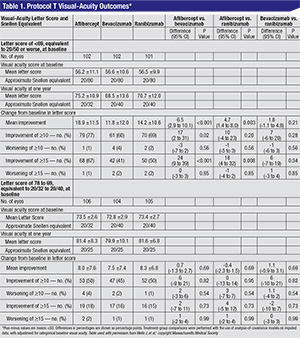
When the initial visual acuity letter score was 78 to 69, or 20/32 to 20/40 Snellen, the mean improvement was eight letters with aflibercept, 7.5 with bevacizumab and 8.3 with ranibizumab (p>0.5), or no significant difference. It was only when researchers stratified the results for worse vision (fewer than 69 letters, equivalent to about 20/50 or worse) that differences started to emerge: the mean improvement was 18.9 with aflibercept; 11.8 with bevacizumab and 14.2 with ranibizumab. (The results and p-values appear in Table 1, p. 39.)
“If a physician took the overall result and said, ‘OK, Eylea’s better,’ and then gave it to everyone, that would ignore the subgroup analysis [in eyes with poorer vision],” says Dr. Wells. “Because in eyes with better vision, all three groups were equal. You’d be overestimating the effect of Eylea in eyes with better vision and underestimating it in the worse eyes.
“A lot of observers might look at the results in the eyes with worsening levels of vision and ask, ‘Nineteen letters vs. 14—are five letters of difference important to patients?’ ” Dr. Wells continues. “That’s misapplying the data. This is a mean difference in two large groups of patients, not a difference for an individual patient. This means that, in eyes with worse vision at baseline, if you were treated with aflibercept, you have a 63-percent greater chance of gaining three lines of vision than if you were treated with bevacizumab, and a 34-percent greater chance than with ranibizumab. So, on a patient level, your chances of gaining three lines of vision were much greater with aflibercept than with the other two in those patients.”
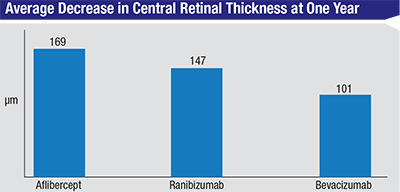
The study also looked at the drugs’ effect on the edema on OCT. At one year, the central subfield thickness decreased, on average, by 169 ±138 µm with aflibercept, 147 ±134 µm with ranibizumab and 101 ±121 µm with bevacizumab. “There was significantly less reduction in OCT thickening with bevacizumab than the other two drugs,” says Dr. Wells. “This didn’t translate into a difference in vision with the eyes with better vision in the first year, so we don’t think it mattered in the first year. Whether it will matter in the second year, we don’t know.”
From Lab to Clinic
Retinal specialists say that the trial was well-done, but to tread carefully when attempting to put the trial data into practice in the clinic.
“The DRCR.net and all the investigators should be congratulated on doing a phenomenal study,” says David Boyer, MD, a clinical professor of ophthalmology at the University of Southern California’s Keck School of Medicine. “However, to put things in perspective, this was one-year data on a chronic disease that we’ll be treating patients for over a long period of time. We’ll find out if the results from the first year hold up in year two or if they change. As a caveat, though, the results in some cases didn’t surprise me. The CATT showed that ranibizumab dried better than bevacizumab, and in the VIEW 1 and VIEW 2 trials aflibercept dried better than ranibizumab, so you’d expect it to be very effective based on these AMD studies.”
“Physicians also have to be aware that the Snellen vision they’re getting in the exam lane is different from the vision measured in the trial,” Dr. Boyer continues. “ETDRS and Snellen are different ways to measure vision; a 20/40 ETDRS may be measured as 20/50 or 20/60 Snellen in some cases. We know that the better visions from the two methods tend to line up more closely, but when you get into the worse visions the spread becomes wider. People have to be aware of this before they take the exact message and translate it over to the clinic. The other aspect that’s important with all of these studies, such as CATT and DRCR.net Protocol T, is that the bevacizumab used in the trials isn’t the bevacizumab that we get from our compounding pharmacies. Instead, it’s specially made and put in glass vials. This is not something you can normally purchase, and could cost $100 to make under sterile conditions. This could impact [impressions of] bevacizumab in a worse way long-term, if physicians use it in a clinic and don’t see the response from the trial.”
Another caveat with the study, surgeons say, is that the trial patients are different from those ophthalmologists see in their daily practice.
“Patients in a study are not necessarily like regular patients,” says Sunir Garg, MD, assistant surgeon at the retina service at Wills Eye Hospital and assistant professor of ophthalmology at Thomas Jefferson University. “Patients in these trials generally tend to be a little bit more healthy, a bit more health-conscious, tend to see their doctors more and are more comfortable around them. So, generally, their level of sugar control and their ability to keep up with their follow-up appointments tend to be better than the patients you see in the clinic during your regular practice. Those differences may influence outcomes, as well.
“The other thing physicians need to remember is that the follow-up and treatment schedule for Protocol T was a pretty regimented schedule,” Dr. Garg adds. “We know that, for diabetics, having a regimented schedule works—at least initially. However, that’s simply one way of doing things. If you look at the original Lucentis and Eylea trials, patients got monthly injections, so we don’t know if even more intensive therapy above and beyond Protocol T would benefit the patients that we saw in Protocol T. Also, all of the Protocol T results are contingent upon giving patients monthly injections for the first six months; regular, everyday patients may not be able to do this. Patients may not be able to get time off from work, they might get sick, they might forget or they might miss an appointment for other reasons. So, all of these things influence what we see day to day, and results may not match up to those in the clinical trial.”
 |
In terms of the study’s impact on physicians’ choices of first-line therapy, doctors say it’s not as clear-cut as simply switching to a different drug for certain patients. Instead, they say, there are several factors to consider.
“I was surprised somewhat by the spread in results in the patients with poorer vision, at least in the first year,” Dr. Boyer says. “I think that, if I usually started a patient on bevacizumab, I’d have to think very hard about the possibility of going to aflibercept in those eyes with poorer vision to dry them out.” Dr. Boyer says that, in addition to the differences between clinical-trial patients and those seen in a clinic as outlined above, economic factors often play a role in the choice of anti-VEGF therapy. “If a patient doesn’t have good insurance, we have to take that into account, but a clinical trial doesn’t,” he says. “In a real-life situation, I don’t think you can fault someone who’s administering a drug that’s inexpensive because the patient can’t afford another one, or may not qualify for a pharmaceutical company’s assistance plan, or is too proud to use the plan. Even if a patient applies for assistance, you don’t want to wait until the application goes through, so you may start with bevacizumab until the application is approved. And, if he responds to bevacizumab, you may keep him on it.”
Dr. Garg says that it’s reasonable to use bevacizumab in patients with unsatisfactory insurance, but doing so evokes nagging doubts about efficacy. “What we don’t know is if you start with Avastin and it doesn’t work, and you subsequently switch to something else, have you lost something?” he muses. “In some of the earlier Lucentis trials for vein occlusion and even diabetes, patients were randomized to either monthly Lucentis injections or, in a sense, laser from the get-go. The patients who started with laser, waited six months and then went to Lucentis, they had improvement but not of the same magnitude as they did if they would have started with Lucentis from day one. It appears that if you just kind of ignore the macular edema, there’s going to be some ‘water damage’—some rotting of the macula, if you will. And when those cells die you’re not going to get them back. So we don’t know if we treat someone with Avastin and they don’t dry up quickly or they still have persistence in their edema in the months before you switch to another drug, if they’re going to do as well as they would have if you had put them on Lucentis or Eylea from the beginning.”
Dr. Garg says the study also helped him look at his injection protocol in a new light. “One of the interesting things about Protocol T is that the OCT outcomes generally mirrored the visual outcomes—as the vision went up, you saw the macular edema go down,” he says. “That wasn’t much of a surprise. But one thing was helpful to me. I was under the impression that I had to keep injecting regularly until every last bit of edema was gone. But in Protocol T, they didn’t do that. They just injected patients monthly until no further reduction of macular edema was present. Then, if they didn’t dry up entirely, you could consider adding focal laser to those patients, and could potentially follow patients with mild residual edema over time without necessarily giving injections.
|
Safety Signals
Many safety measures were well within acceptable limits in Protocol T, such as lines of vision lost and injection-related events. A cardiovascular risk emerged at one point, but physicians don’t think it jibes with the body of evidence on anti-VEGF.
At one year, Dr. Wells says less than 2 percent of eyes lost 15 or more letters of vision, and vascular events occurred at rates of 3 percent in the aflibercept group, 4 percent in the bevacizumab group and 5 percent in the ranibizumab group (p=0.56). When the researchers performed a post hoc analysis, though, the frequency of events in the combined Medical Dictionary for Regulatory Activities system organ classes of cardiac and vascular disorders was higher in the ranibizumab group (p=0.01 excluding hypertension events and p=0.04 including those events). “We debated even including this finding,” says Dr. Wells, “but we had to because we had found it. We really think it was due to chance, because we had never seen things like that in other studies of ranibizumab. Also, because it was a post hoc analysis, it has less statistical strength than a pre-specified one.” Dr. Boyer doesn’t think the post hoc safety data is concerning, either. “To be honest, I don’t put much credence in that,” he says. “In every trial, we have these inconsistencies where one, two or three events may drive the whole study. I think all the drugs are safe.”
All things considered, Dr. Garg says he appreciates the findings of Protocol T. “To me, as a practicing retina physician out in the trenches, this is one of the more important studies to come out in a while,” he says. “I mean this not only from a pie-in-the-sky perspective, but in day-to-day terms of how to treat patients and work through the clinical questions we constantly grapple with. Protocol T has been very illuminating for a lot of us.” REVIEW
1. Wells J, Glassman A, Ayala A, The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab or ranibizumab for diabetic macular edema. N Engl J Med 2015;26;372:13:1193-203.