For millennia, humans have searched for and developed methods of controlling pain. The pain- and fever-reducing qualities of willow bark have been well-documented over the years, as they were first described in the Hippocratic Corpus around 400 B.C. Henri Leroux, a French pharmacist, and the Italian chemist Raffaele Piria isolated the active component salicin from willow bark in 1828.1
However, it wasn't until 1897 that a chemist at the fledgling German pharmaceutical company Bayer created acetylsalicylic acid. Through a reaction of salicylic acid and acetic anhydride,2 non-steroidal anti-inflammatory drugs first came into modern pharmaceuticals. Many other NSAIDs have since been developed, and although not all are salicylates, their mechanism of action is similar: They inhibit the enzyme cyclooxygenase, which catalyzes the conversion of arachidonic acid to prostaglandins and thromboxane.
The arachidonic acid cascade was not elucidated until 1971,3 but NSAIDs—both prescription and over-the-counter—have been extensively used for millennia to treat pain and inflammation. According to a poster presented by
The Arachidonic Acid Cascade
Arachidonic acid is the precursor to prostaglandins, thromboxanes and leukotrienes. There are two confirmed paths arachidonic acid can take. When arachidonic acid is oxygenized by 5-lipoxygenase, leukotrienes are synthesized. Leukotrienes are involved in neutrophil recruitment and inflammation, and are therefore important to the pathology of asthma and allergy, as well as the body's immune response to infection. The cyclooxygenase enzyme synthesizes the reaction between arachidonate acid and prostaglandin PGG2 and the subsequent reduction of PGG2 to PGH2. PGH2 can then be converted into various prostaglandins and thromboxane. While COX-1 maintains normal levels of prostaglandins, COX-2 increases the production of prostaglandins, thereby enhancing the inflammatory response. Traditional NSAIDs interfere with the functioning of COX-1 and COX-2, and have no direct effect on the path induced by 5-lipoxygenase.
Gastric ulcers are an unpleasant by-product of excessive COX-1 inhibition, and is the subject of a warning associated with traditional systemic NSAIDS. In recent years, selective COX-2 inhibitors were developed to avoid this; however, they increased the risk of cardiovascular events.
Side Effects
Although side effects can occur with the use of any drug, some of the adverse events that have been attributed to NSAID use only occur in the presence of pre-existing conditions or other complicating factors. These include:
• Systemic. Patients using topical ophthalmic medications tend not to develop systemic effects of the drug. Systemic effects of topical NSAIDs are mostly related to drainage from the eye through the nasal-lacrimal duct. Although several instances of exacerbation of asthma in connection with the use of ophthalmic NSAIDs have been reported, most were in patients with histories of NSAID hypersensitivity or asthma.8-11 One patient had received diclofenac (Voltaren Ophthalmic), and punctal plugs prevented subsequent attacks.8
Individuals with asthma sometimes experience exacerbation of asthma symptoms with the use of aspirin or other systemic NSAIDs; this is hypothesized to be the result of a shifting effect caused by COX inhibition. NSAID-induced COX inhibition may increase the amount of arachidonic acid that is available for the creation of leukotrienes,12 which in turn initiates asthma symptoms in susceptible individuals. Similarly, local side effects associated with ophthalmic NSAIDs tend to correspond to use in individuals predisposed to such conditions.
• Surge in melting. Prior to 1999, ophthalmic NSAIDs were used with little concern about side effects. NSAIDs are considered to be a standard treatment for inflammation and pain following cataract and other ophthalmic surgeries. The most common side effects of topical NSAIDs are minor: burning; stinging; and conjunctival hyperemia.13 Complications reported to be the result of ophthalmic NSAID use include keratitis, corneal infiltrates and corneal lesions; however, similar complications are also associated with topical ophthalmic medications that contain preservatives.14 With the introduction of a generic version of diclofenac sodium ophthalmic solution (Falcon Pharmaceuticals) in 1998,15 an increase in severe ocular adverse events—particularly corneal stromal ulceration—was noticed by ophthalmologists. More sensationally known as corneal melting, these "melting ulcers" progressively overtake the cornea. Melts can't occur without epithelial defects, and are thus linked to cataract surgery, refractive surgery and contact lens irritation. Indeed, melts are known to occasionally occur in these conditions (and many others) without the use of NSAIDs.16,17 If the cornea doesn't reepithelialize, it's left open to infection. In response, immune cells and collagenases attack the pathogens and the infected tissue, resulting in inflammation and the formation of ulcers. Postoperatively, chronic inflammation along the limbus corresponds with the development of melting ulcers. To our knowledge, and with the possible exception of generic diclofenac, corneal melting has not been reproduced in laboratory animals through the use of NSAIDs. Ophthalmic NSAIDs are neither a primary or typical cause of melts.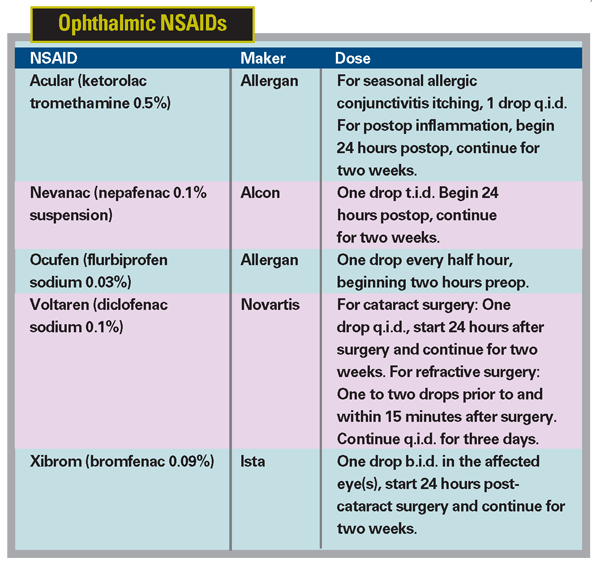
Although the etiology of stromal ulcers is a web of many different environmental and pathological influences, the generic diclofenac was quickly recalled. Questions remain as to why generic diclofenac was associated with so many more cases of corneal melting than Voltaren, the brand name version of diclofenac ophthalmic manufactured in the United States by Novartis Ophthalmics.18 Both generic diclofenac and Voltaren consisted of 0.1% diclofenac sodium, but their inactive ingredients, the buffers, solubilizers and preservatives, were entirely different. At least one group of researchers has suggested that the inactive ingredients of generic diclofenac may have been involved in the pathogenesis of the stromal ulcers.18 The solubilizer used in generic diclofenac is tocophersolan; tocopherols have been demonstrated to inhibit retinal pigment epithelial cell proliferation19 and to induce apoptosis in mouse mammary cells.20 Unfortunately, the exact relationship, if any, between these "inactive" ingredients and corneal melts remains unclear.
• Wound healing. Similarly enigmatic is the proposed connection between matrix metalloproteinases and NSAIDs. MMPs are a family of enzymes that degrade extracellular matrix proteins, and are intimately involved in tissue remodeling.21 Of course, their effects aren't always beneficial, and MMPs are also involved in metastasis, arthritis and cirrhosis. In rats with thermally induced corneal wounds, increased levels of MMPs have been observed; inhibition of MMP activity has been noted to improve basement membrane repair.22 Increased MMP activity was also measured in at least a couple of cases, including a poster from the University of Bombay at the 2000 meeting of the American Academy of Ophthalmology, in which stromal ulcers in patients who underwent corneal surgeries were being treated with NSAIDs.18,23 Further studies have suggested a correlation between corneal ulcers, NSAIDs and increased MMP activity,23 though irrefutable evidence is elusive.
In any event, the possibility of NSAID-induced corneal melting resulted in a question that's still debated today: Do NSAIDs delay wound healing? The answer may depend on which NSAID is being used. Diclofenac is unique in that it indirectly affects the lipoxygenase pathway. It enhances the uptake of arachidonic acid into triglyceride pools, thereby inhibiting keratocyte proliferation and increasing the risk of delayed wound healing and corneal ulceration.24 The concentration and dosing frequency of the agent also impacts adverse events.
On the other hand, studies conducted with animal models suggest that NSAIDs have the opposite effect on wound healing. A cat model (with ketorolac tromethamine 0.5%) of corneal and limbal wound healing showed that NSAIDs may actually enhance wound healing and reduce the risk of postoperative complications.25 Furthermore, the location of the wound affects its healing. Because the limbus is surrounded by blood and lymphatic vessels, limbal wounds heal more rapidly than corneal wounds. Although animal models often lack clinical relevance, the positive effect of NSAIDs on corneal wound healing in animals strongly suggests that any deleterious effects noticed in humans are not due to NSAIDs, but rather to co-existing conditions.
Solid History of Efficacy
Although the etiology of NSAID-associated side effects is subject to close scrutiny, the efficacy of NSAIDs is well-established. NSAIDs are effective analgesics with mild anti-inflammatory capabilities. Their effects are often preferred over those of opioids, whose anesthetic/sedative effects can disrupt normal ocular functioning. Ophthalmic NSAIDs are therefore used for the treatment and prevention of an array of conditions, including postoperative inflammation, intraoperative miosis, allergic conjunctivitis and pain after refractive surgery. They are also used off-label for the prevention of postop cystoid macular edema.
Within this diverse patient population, there exist many risk factors for NSAID-associated adverse events. Autoimmune diseases, such as rheumatoid arthritis and Sjögren's syndrome, bacterial infections, chronic dry eye and rosacea are common afflictions that are associated with the formation of corneal ulcers. Diabetes is also considered to be a risk factor for ophthalmic NSAID-associated side effects. Steroids are known contributors to ulcer pathogenesis, and use alongside of NSAIDs is strongly discouraged. Contact lens wear is perhaps one of the most common causes of corneal infiltrates and ulcers,26 as overuse and ineffective cleaning of the lenses can cause corneal abrasions and introduce bacteria to the ocular surface. Given the prevalence of these conditions and factors, along with the popularity of NSAIDs, it's not surprising that NSAIDs have been correlated with undesirable effects.
As with any drug, long-term use of ophthalmic NSAIDs exposes patients to the risk of adverse events. Persistent suppression of prostaglandins is reported to cause a compensatory increase in the production of leukotrienes. Although leukotrienes likely have limited roles in the conjunctiva,27 they are present in the cornea, including the corneal epithelium.28 There they are involved in enhancing leukocyte chemotaxis and infiltration, along with vascular permeability. In the setting of an epithelial defect that won't heal, prolonged use of topical NSAIDs could facilitate the development of corneal ulcers. In addition, long-term suppression of prostaglandins robs the cornea of its normal housekeeper, delaying response to infections. Prolonged use of NSAIDs should be conducted with caution.
All drugs have side effects. Despite all their possible sources, the frequency of side effects associated with ophthalmic NSAIDs is low in relation to the number of patients treated annually with them.29 Ophthalmic NSAIDs have an impressive safety and efficacy profile stretching back more than 20 years. Most often, side effects are related to mis- or overuse of the drug. The hype and panic over corneal melting associated with NSAIDs, particularly with generic diclofenac, appears to be at least partially related to misinterpretation of cause-and-effect relationships. Adverse events are not known to occur during the use of ophthalmic NSAIDs without high and/or prolonged dosing, corneal epithelial defects, specific NSAIDs or predisposing systemic conditions.
Nevertheless, the possibility remains that NSAIDs—alone or in concert with pre-existing conditions—can cause undesirable side effects. Clinicians must not indiscriminately prescribe NSAIDs to every patient after cataract or refractive surgery, but take careful exams and histories to ensure effective treatment without side effects.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Marson P, Pasero G. The Italian contributions to the history of salicylates. Reumatismo 2006; 58:1:66-75.
2. Hoffman H. US Patent 644,077 dated February 27, 1900. US Patent Office.
3. Vance JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 1971;231:25:232-5.
4. Colin J, Paquette B. Comparison of the analgesic efficacy and safety of nepafenac ophthalmic suspension compared with diclofenac ophthalmic solution for ocular pain and photophobia after excimer laser surgery. Clin Ther 2006;28:4:527-36.
5. Uchio E, Itoh Y, Kadonosono K. Topical bromfenac sodium for long-term management of vernal keratoconjunctivitis. Ophthalmologica 2007;221:3:153-8.
6. Simone JN, Pendelton RA, Jenkins JE. Comparison of the efficacy and safety of ketorolac tromethamine 0.5% and prednisolone acetate 1% after cataract surgery. J Cataract Refract Surg 1999;25:5:699-704.
7. Tauber J,
8. Sharir M. Exacerbation of asthma by topical diclofenac. Arch Ophthalmol 1997;115:294-5.
9. Polachek J, Shvartzman P. Acute bronchial asthma associated witih the administration of ophthalmic indomethacin. Isr J Med Sci 1996;32:1107.
10. Sheehan GJ, Kutzner MR, Chin WD. Acute asthma attack due to ophthalmic indomethacin. Ann Intern Med 1989;111:337-8.
11. Sitenga GL, Ing EB, van Dellen RG, Younge BR, Leavitt JA. Asthma caused by topical application of ketorolac. Ophthalmology 1996;103:6:890-2.
12. Piper PJ. Formation and actions of leukotrienes. Physiol Rev 1984;64:744-61.
13. Flach AJ. Cyclo-oxygenase inhibitors in ophthalmology. Surv Ophthalmol 1992;36:259-84.
14. Wilson FM. Adverse external ocular effects of topical ophthalmic therapy: An epidemiologic, laboratory, and clinical study. Trans Am Ophthalmol Soc 1983;81:854-965.
15. Diclofenac sodium ophthalmic solution, 0.1% package insert. Falcon Ophthalmics, Inc. Forth Worth, TX. March 1998.
16. Foster CS. Immunologic Disorders of the Conjunctiva, Cornea, and Sclera. In: Albert DM, Jakobiec FA, eds. Principles and Practice of Ophthalmology, 2nd Ed. Philadelphia: WB Saunders Company; 2000:803-828.
17. Kenyon KR, Yoo SH, Starck T, Wagoner MD. Corneal Epithelial Defects and Noninfectious Ulcerations. In: Albert DM, Jakobiec FA, eds. Principles and Practice of Ophthalmology, 2nd Ed. Philadelphia: WB Saunders Company; 2000:926.
18. Hargrave SL, Jung JC, Fini ME, et al. Possible role of the vitamin E solubilizer in topical diclofenac on matrix metalloproteinase expression in corneal melting: An analysis of postoperative keratolysis. Ophthalmology 2002;109:343-50.
19. Sakamoto T, Hinton DR, Kimura HK, et al. Vitamin E succinate inhibits proliferation and migration of retinal pigment epithelial cells in vitro: Therapeutic implication for proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol 1996;234:186-92.
20. McIntyre BS, Briski KP, Gapor A, Sylvester PW. Antiproliferative and apoptotic effects of topcopherols and tocotrienols on preneoplastic and neoplastic mouse mammary epithelial cells. Proc Soc Exp Biol Med 2000;224:292-301.
21. Stolow MA, Bauzon DD, Li J, et al. Identification and characterization of a novel collagenase in Xenopus laevis: Possible roles during frog development. Mol Biol of the Cell 1996;7:1471-83.
22. Fini ME, Parks WC, Rinehart WB, et al. Role of matrix metalloproteinases in failure to re-epithelialize after corneal injury. Am J Pathology 1996;149:4:1287-1302.
23. O'Brien TP, Li QJ, Sauerburger F, Reviglio VE, Rana T, Ashraf MF. The role of matrix metalloproteinases in ulcerative keratolysis associated with perioperative diclofenac use. Ophthalmology 2001;108:4:656-9.
24. Ku EC, Lee W, Kothari HV, Scholer DW. Effect of diclofenac sodium on the arachidonic cascade. Am J Med 1986;80(Suppl 4B):18-23.
25. Barba KR, Samy A, Lai C, Perlman JI, Bouchard CS. Effect of topical anti-inflammatory drugs on corneal and limbal wound healing. J Cataract Refract Surg 2000;26:893-897.
26. Poggio EC,
27. Absleon MB. Lipooxygenase products in ocular inflammation (abstract). Invest Ophthalmol Vis Sci 1984;25(S):42.
28. Weston JH,
29. Congdon NG, Schein OD, von Kulajta P, et al. Corneal complications associated with topical ophthalmic use of nonsteroidal antiinflammatory drugs. J Cataract Refract Surg 2001;27:4:622-31.



