Ophthalmologists make decisions every day about whether to prescribe a solution, suspension or ointment; whether the route of administration should be topical, injected or systemic; or whether the dosing regimen can be once-daily, more than once a day or through an extended-release platform. These decisions are as key to the treatment plan as the choice of medication, and are informed by a fundamental understanding of ocular pharmacokinetics (PK).
This month we examine the principles that underlie these therapeutic decisions. In an upcoming column we’ll examine how the modulation of drug PK behavior has become a factor in the development of new drugs.
Pharmacokinetics Explained
Pharmacokinetics seeks to understand what the body does to a drug in order to establish a quantitative relationship between administered dose and dosing regimen and the concentration of the drug in plasma and/or in tissue.1 This relationship allows maximal efficacy to be balanced against minimal toxicity. The studies that provide PK data for drug approvals are strictly controlled and provide a generalizable result to guide prescribing physicians. Given that each patient who presents in the clinic is unique and has a disease etiology that’s likely to be unique as well, the treating physician must be prepared to apply his understanding of how the drug works to each case.
As the name implies, pharmacokinetics is about time, but it is also about dose, and the relationship between the two. For every drug or formulation, there is a relationship between dosing and drug exposure. The sum total of that exposure, or the “area under the curve” of the concentration versus time curve, dictates that exposure and the opportunity for drug efficacy.
All drugs targeting ocular tissues, regardless of their route of administration, must contend with anatomical and physiological barriers to deliver a safe and effective dose to the target tissue.2,3 How those drugs overcome those barriers has implications for patient adherence to treatment, which, in turn, affects treatment success.
Systemic Administration
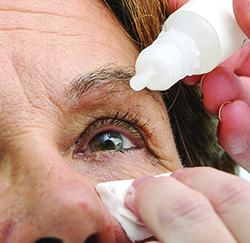 |
| If a patient can suppress her blinking for four to six seconds prior to instilling a drop, the breakup of the tear film that results can give the drop better access to the ocular surface. |
Systemically administered ophthalmic drugs benefit from high patient treatment adherence due to the relative simplicity of the dosing regimen in terms of frequency, ease of administration and non-invasiveness.2,4 Despite this considerable benefit, systemically administered drugs must compensate for the loss in concentration experienced during absorption, metabolism, distribution and excretion. Consequently higher dosages of the drug have to be administered, which also increases the risk of systemic toxicity, making the option less desirable.5
Topical Administration
At a weight of approximately 8 g, the eye is a relatively small target for systemic drug delivery.6 Topical ocular drugs have the advantage of directly targeting the eye, while avoiding first-pass metabolism. Despite this, other sophisticated barriers exist to impede delivery of drug to ocular tissue.
Upon instillation, topical ocular drugs must contend with spillage, dilution, blinking and drainage, as well as basal and reflex lacrimation. The eye can hold approximately 10 to15 μl, which is considerably smaller than the typical eye drop volume of 40 μl. This imbalance results in an initial loss of drug to overflow. Any drug remaining on the surface is diluted by the tear film where albumin and other proteins may bind to the drug, further reducing drug concentration. Within a few minutes, the healthy tear film turns over, replacing itself entirely, so whatever drug is not absorbed by the cornea and conjunctiva drains down the nasal lacrimal duct. Consequently, the contact time of the drug with the cornea, conjunctiva and sclera is brief.
The cornea is the primary path for drugs to penetrate from the tear film to the anterior segment. The corneal epithelium is highly lipophilic, posing a significant barrier to topically administered hydrophilic drugs, and the superficial epithelial cells are surrounded by tight junctional complexes that permit only small-molecule drugs to permeate transcellularly from the tear film.2,7,8 The stroma is the next barrier, and it is hydrophilic, restricting further permeation of highly lipophilic drugs passing from the epithelium. Additionally, enzymes present in the cornea act to metabolize drugs before they further penetrate into the eye. The combination of precorneal and corneal barriers results in less than 5 percent of the instilled drug penetrating the cornea and reaching intraocular tissues.9
Though the conjunctiva also has tight junctions, the intercellular spaces are slightly larger than those in the cornea.3 While this allows better penetration by larger molecules, the presence of conjunctival blood capillaries lowers bioavailability of drug through elimination via systemic blood circulation.2
Drug that permeates these barriers must continue through the blood-aqueous barrier, which is composed of the endothelial cells in the uvea. Similarly to the cornea and conjunctiva, the blood-aqueous barrier is controlled by tight junctions. Drug that diffuses into the aqueous humor is then eliminated by aqueous turnover and by the blood flow of the anterior uvea.
Due to the direct targeting, eye drops result in a higher bioavailability than systemically administered drugs, especially in the anterior chamber. Drops that require no more than q.d. or b.i.d. dosing promote patient treatment adherence through a simplified treatment regimen, lower toxicity and fewer side effects.9 However, if instillation of a drop is challenging or if it’s associated with transient effects such as stinging or blurring of vision upon instillation, treatment adherence can be detrimentally affected.
Injections
Topical eye drops can effectively impact anterior segment targets, but barriers limit their ability to reach posterior segment targets. As a result, delivery of drug to posterior segment targets often involves subconjunctival, sub-Tenon’s and peribulbar injection or comparatively more invasive administration via intravitreal injection. These methods avoid many of the barriers posed by topical and systemic routes, but effective delivery of the drug in this manner isn’t obstacle-free.
Drug can be injected into the subconjunctival, sub-Tenon’s or peribulbar space to create a depot for extended drug release, which bypasses the barriers posed by the tear film and corneal-conjunctival barriers. Upon release, however, the drug must pass through the sclera, choroid and RPE and it must contend with elimination by blood and lymphatic circulation, which rapidly lowers the bioavailability of the drug.10,11 Drugs injected intravitreally must diffuse through the vitreous to the retina, where they must permeate the retina and the RPE to reach the choroid. The retina limits the passage of macromolecules, while the tight junctions of the RPE further restrict passage of hydrophilic compounds.3,12 Larger molecules are primarily eliminated via the annular gap between the lens and the ciliary body, while smaller or lipophilic molecules are eliminated by the retina-choroid-sclera membrane.13
When compared to systemic and topical delivery, periocular and intravitreal injections are efficient methods of delivering drug to the posterior segment at sustained drug levels.2 But while these methods require fewer doses over time, they are associated with more complications, such as subconjunctival hemorrhages, endophthalmitis, traumatic cataract, rhegmatogenous retinal detachment and RPE tears associated with intravitreal injections.14 These complications can discourage patient treatment adherence when prolonged treatment regimens of repeated doses are required.
Treatment Considerations
Bioavailability of drug in the eye is negatively affected by barriers to penetration, drainage, dilution and metabolism. To overcome these obstacles the clinician needs to consider methods to improve bioavailability by selecting drugs or treatment methods that enhance penetration to the target tissue or that increase dwell time. Patient acceptance of the chosen drug and treatment method is a significant consideration as well. If the safety profile of a drug raises patient concerns about its risk/benefit ratio, or if the treatment regimen is too complicated or challenging, then treatment adherence is at risk and may render the most effective drug ineffective in practice.
Historically, topical ocular drugs have been the most convenient treatment for anterior chamber diseases, with choices ranging from solutions, emulsions and suspensions to ointments and gels.7 Compared to solutions, emulsions and suspensions, ointments and gels increase bioavailability through prolonged ocular contact time. However, the increased viscosity tends to also lead to temporary discomfort and transient blurring of vision. Even temporary and minor side effects such as these can reduce patient adherence.
Within the last decade researchers have explored several novel drug-delivery approaches aimed at reducing the need for frequent dosing. Prodrugs and cyclodextrins have been studied as a means to improve ocular penetration of drug, while various colloidal delivery systems have been studied as a means to prolong the duration of drug action.7 These developments are significant and are likely to change how we treat ocular disease. We will explore these developments in the near future.
As researchers continue to look for effective ways to deliver drugs to target tissues, there are some practical tips for the clinician to consider when prescribing topical drops. First, consider manual punctal occlusion. Since a significant amount of drug in an eye drop drains through the nasolacrimal ducts, manually occluding the puncta increases contact time of the drug with the ocular surface and minimizes systemic absorption, which is an important consideration where systemic side effects are of concern. Second, consider recommending the patient hold the eye open for a few seconds before administering a drop. This allows the average tear film to break apart, reducing the effectiveness of the pre-corneal barrier and allowing the drug to penetrate more quickly. Third, properly instruct patients on how and why they should instill their medication. Treatment adherence is not only taking medication when one is supposed to but also taking it in the specified manner. Eye drops can be challenging to self-administer, especially for elderly patients. This may result in the unintended result of patients taking more than the specified number of drops. Patients may also believe that taking more drops is better. In both instances, given the restricted volume of the lacrimal lake, additional drops are wasteful and could lead to contact dermatitis from excess spillage. Also, if more than one topical ocular drug is prescribed, patients should wait several minutes before instilling the second medication to avoid washing out the first.
Treatment success is often a matter of drug selection and treatment adherence. Ophthalmologists who understand basic ocular pharmacokinetics can make the most informed choices about drug formulation, mode of instillation and dosing schedule as they relate to the specific requirements of the patient. REVIEW
Dr. Abelson is a clinical professor of ophthalmology at the Harvard Medical School, and emeritus surgeon at the Massachusetts Eye and Ear Infirmary.
1. Worakul N, Robinson JR. Ocular pharmacokinetics/pharmacodynamics. Eur J Pharm Biopharm 1997;44:1627:71.
2. Gaudana R, Ananthula HK, Parenky A, et al. Ocular drug delivery. AAPS J 2010;12:3:348-60.
3. Kim YC, Chiang B, Wu X, et al. Ocular delivery of macromolecules. J Control Release 2014;28:190:172-81.
4. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:5:487-97.
5. Kaur IP, Smitha R, Aggarwal D, et al. Acetazolamide: Future perspective in topical glaucoma therapeutics. Int J Pharm. 2002;248:1-2:1-14.
6. Todd TW, Beecher H, Williams GH, et al. The weight and growth of the human eyeball. Hum Biol 12:1940:1-20.
7. Cholkar K, Patel SP, Vadlapudi AD, et al. Novel strategies for anterior segment ocular drug delivery. J Ocul Pharmacol Ther 2013;29:2:106-23.
8. Hornof M, Toropainen E, Urtti A. Cell culture models of the ocular barriers. Eur J Pharm Biopharm 2005;60:2:207-25.
9. Bourlais CL, Acar L, Zia H, et al. Ophthalmic drug delivery systems – recent advances. Pro Retin Eye Res 1998;17:1:33-58.
10. Ranta VP, Mannermaa E, Lummepuro K, et al. Barrier analysis of periocular drug delivery to the posterior segment. J Control Release 2010;7:148:42-8.
11. Hosseini K, Matsushima D, Johnson J, Widera, Nyam K, Kim L, Xu Y, Yao Y, Cormier M. Pharmacokinetic study of dexamethasone disodium phosphate using intravitreal, subconjunctival, and intravenous delivery routes in rabbits. J Ocul Pharmacol Ther 2008;24:3:301-8.
12. Cuhna-Vaz JG. The blood-ocular barriers: Past, present, and future. Doc Ophthalmol 1997;93:1-2:149-57.
13. Tojo KJ, Ohtori A. Pharmacokinetic model of intravitreal drug injection. Math Biosci 1994;123:1:59-75.
14. Hasler PW, Bloch SB, Villumsen J, et al. Safety study of 38,503 intravitreal ranibizumab injections performed mainly by physicians in training and nurses in a hospital setting. Acta Ophthalmol 2015;93:2:122-5.



