Ocular Pigments
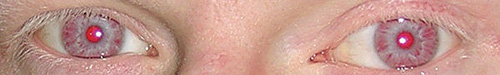
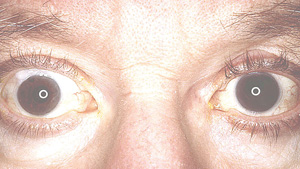
There are two major pigmented structures in the eye, the iris and the retinal pigment epithelium. While RPE cells are central to overall retinal function, here we will focus on the pigments found in the iris. The iris is composed of two layers: a stromal, anterior layer and a posterior pigmented epithelial layer.1,2 The two layers both contain pigmented cells but are embryologically distinct. The stromal layer cells are derived from the neural crest, while posterior epithelial cells are ectodermal in origin. The melanocytes of both layers contain melanosomes, specialized organs that synthesize and store various melanins. There is no compelling evidence suggesting any of these pigmented structures are ever secreted from the melanocytes in the eye, although this is common in other tissues. What we refer to as eye color derives from a combination of factors, including the types of melanins synthesized, the pigments of the anterior iris and the light scattering properties of the anterior stroma.
The two major pigments synthesized in all melanocytes are the eumelanin (black/brown) and pheomelanin (red/yellow).3,4
|
The mechanics of pigment production rely on a family of related enzymes, the tyrosinases.5 Two major family members, tyrosinase and tyrosinase-related protein 1, catalyze oxidations including the key conversion of tyrosine to dihydroxyphenylalanine. Several additional oxidation steps follow to ultimately generate both eumelanin and pheomelanin. The difference between the two pigments is that pheomelanin also includes cysteine as one of its building-block components, and so the availability of this precursor can also impact iris color. Expression levels of TYR and TYRP are critical to pigment synthesis, and genetic defects in their expression result in two types of oculocutaneous albinism, OCA1 and OCA3.
Genetics of Eye Color
The presentations of some forms of pigment disorders are striking, and so they may seem more common conditions than they really are; in the United States about 1:16,000, or 20,000 individuals, have some form of albinism.6 The specific genotypes dictate the spectrum of disease and impact on visual function. Genes implicated in the four major types of oculocutaneous albinism, OCA1 to -4, have been identified, and an understanding of their function has provided important clues to the role of pigmented cells in the eye.7
The most common form of the disease, OCA1, is caused by mutations in the TYR gene, and it results in either a partial or complete loss of melanin biosynthesis.6,7 These patients have pinkish skin, blue-grey irides and a prominent photophobia. They typically have poor acuity, foveal hypoplasia, nystagmus and strabismus. Melanocytes are known to play extensive roles in development, and this may underlie both foveal defects and less common instances of chiasmic misrouting of nerve fiber tracts. Recent work has focused on correlating foveal organization and BCVA to try and understand the role iris pigmentation plays in ocular development.
Less profound types of albinism, including OCA2 and OCA4, are caused by defects in melanosome function that result in low amounts of eumelanin.6,7 This shift in the ratios of pigments has subtle effects, both in terms of the affected individual’s appearance, and in the extent of visual function impairment. Some pigments are visible at birth, and any color in skin and eyes increases with age. Ocular issues such as nystagmus and photophobia are present but less severe than in OCA1. The mutations associated with the OCA4 gene are most common in patients with a Japanese heritage. Mutations in the TYRP gene are classified as OCA3, and are most common in those of African ancestry. Affected individuals display reduced pigmentation and lighter-colored eyes, and have the mildest forms of nystagmus and strabismus.
Melanosome Control
One of the features of the melanosome that distinguishes it from other intracellular structures is an enzyme complex that includes TYR, TYRP and the melanocyte-stimulating hormone receptor.2,4 This complex responds to MSH and other signaling molecules by increasing functional expression of TYR, leading to an increase in melanin synthesis. Melanins have several roles, but key among these is protection from the harmful effects of UV radiation, and both UVA and UVB wavelengths are readily absorbed by both pigments.
|
Patients using PGE2-containing formulations to enhance growth of eyelashes may also experience changes in the color and shape of the upper eyelids. This is due to the stimulation of skin pigment expression, and a reduction in the volume of fat deposits, an effect also stimulated by PGE2.10 The resulting sunken ocular sulcus, particularly on the upper lids, is an effect that most patients will not be happy with. While some might make the aesthetic choice between longer lashes or sunken sockets, for those using PG agonists to treat ocular hypertension, it may be possible to mitigate this effect by switching to a different product.10
Disease Risk/Drug Interactions
Our discussion of eye color goes beyond genetics and aesthetics. There have been many studies published with the goal of correlating eye color with the risk of various ocular disorders, from melanomas to age-related macular degeneration.11-13 There is no shortage of such risk assessments, and they do provide some information on the relationship between eye color and relative risks. For example, several studies have shown positive correlation between iris pigment and the risk of geographic atrophy or glaucoma. Similarly, the increased risk of ocular melanoma in those with lighter eye color is similar to other melanomas for which those with lighter skin are at greater risk of developing the disease. Despite these findings, focusing on epidemiological trends may overlook the greater potential impact of ocular pigmentation on drug pharmacokinetics.
|
Patients using PGE2-containing formulations to enhance growth of eyelashes may also experience changes in the color and shape of the upper eyelids.
|
This binding activity is seen across multiple classes of drugs, yet is predominantly drug-specific: For example, triamcinolone binds with high affinity, while dexamethasone does not. The importance of melanin binding is demonstrated by its inclusion as one of the required tests in the toxicological profiling of topically applied drugs.
Eye color is a trait we share with other higher vertebrates, yet it seems a very human attribute. While we can classify a person according to a predominant coloration, the patterns and intricacy of the iris help to remind us of our individuality. They can also serve as a reminder of each of our patient’s unique (and not always predictable) response to their therapeutic regimes. REVIEW
Dr. Abelson is a clinical professor of ophthalmology at Harvard Medical School. Dr. McLaughlin is a medical writer at Ora Inc.
1. Yamaguchi Y, Hearing VJ. Melanocytes and their diseases. CSH Perspectives in Med 2014;4a017046.
2. Sturm RA, Frudakis TN. Eye colour: Portals into pigmentation genes and ancestry. Trends Genetics 2004;20:327-332.
3. Simon JD, Hong L, Peles DN. Insights into Melanosomes and Melanin from Some Interesting Spatial and Temporal Properties. J Phys Chem B 2008;112:13201-13217.
4. Schiaffino MV. Signaling pathways in melanosome biogenesis and pathology. Int J Biochem Cell Biol 2010;42:1094-1104.
5. Ray K, Chaki M, Sengupta M. Tyrosinase and ocular diseases: Some novel thoughts on the molecular basis of oculocutaneous albinism type 1. Prog Retin Eye Res 2007;26:323-358.
6. Levin AV, Stroh E. Albinism for the busy clinician. J AAPOS 2011;15:59-66.
7. Rennie IG. Don’t it make my blue eyes brown: Heterochromia and other abnormalities of the iris. Eye 2012;26:29-50.
8. Stjernschantz JW, Daniel M, Albert DM, Hu D-N, Drago F, Wistrand PJ. Mechanism and clinical significance of prostaglandin-induced iris pigmentation. Surv Ophthalmol 2002;47:S162–S175.
9. Chou S-Y, Chou C-K, Kuang T-M, Hsu W-M. Incidence and severity of iris pigmentation on latanoprost-treated glaucoma eyes. Eye 2005;19:784-787.
10. Sakata R, Shirato S, Miyata K, Aihara M. Recovery from deepening of the upper eyelid sulcus after switching from bimatoprost to latanoprost. Jpn J Ophthalmol 2013;57:179-184.
11. Yonekawa Y, Kim IK. Epidemiology and management of uveal melanoma. Hematol Oncol Clin North Am 2012;26:1169-84.
12. Mitchell R, Rochtchina E, Lee A, Wang JJ, Mitchell P; Blue Mountains Eye Study. Iris color and intraocular pressure: The Blue Mountains Eye Study. Am J Ophthalmol 2003;135:384-6.
13. Fraser-Bell S, Choudhury F, Klein R, Azen S, Varma R; Los Angeles Latino Eye Study Group. Ocular risk factors for age-related macular degeneration: The Los Angeles Latino Eye Study. Am J Ophthalmol 2010;149:735-40.
14. Beberok A, Buszman E, Wrześniok D, Otręba M, Trzcionka J. Interaction between ciprofloxacin and melanin: The effect on proliferation and melanization in melanocytes. Eur J Pharm 2011;669:32–37.
15. Du W, Sun S, Xu Y. et al. The effect of ocular pigmentation on transscleral delivery of triamcinolone acetonide. J Ocul Pharmacol Ther 2013;29:633-8.